Oral Presentation International Solvent Extraction Conference 2025
Discussing the Potential of Novel Solvents for Acetic Acid Recovery from Gas Fermentation Processes via Reactive-Extraction (122627)
Scope and Motivation:
Research on the autotrophic microbial transformation of carbon-rich off-gases into organic compounds has greatly accelerated since LanzaTech commercialized ethanol production via gas fermentation [1]. A promising alternative product from gas fermentation processes is acetate, the anion of acetic acid (HAc), expanding the spectrum of products achievable from CO₂ and H₂ through biological pathways. However, unlike ethanol, which is easily separated from water by conventional distillation methods, HAc cannot be efficiently separated by simple distillation due to its high boiling point (118 °C) and a relative volatility close to one [2]. Alternatively, extraction methods are promising low-energy unit operations to recover acetic acid from fermentation broths [3].
Additionally, the gas fermentation process requires pH adjustment to protonate pH-neutrally produced acetate for subsequent downstream processing. Previous studies have shown that electrochemical pH management based on water splitting induced by an electric current is a cost- and resource-efficient alternative to conventional acid and base addition [4, 5]. However, an electrochemical approach requires a background electrolyte such as sodium sulfate (Na₂SO₄), thus necessitates highly selective separation methods to isolate HAc from (a) water and (b) the electrolyte. Therefore, we focus on a process strategy to recover HAc from gas fermentation broth combining electrochemical pH pretreatment with subsequent selective reactive extraction [6].
In reactive extraction, typical extractants used for carboxylic acid recovery are, e.g., trioctylphosphine oxide (TOPO) and trioctylamine (TOA) [7, 8]. In this contribution, we address the challenge of maximizing selectivity for HAc extraction over Na₂SO₄ and water by tailoring organic reactive extraction systems containing TOPO and TOA and optimizing operating conditions. We explore the use of terpenoids (menthol, thymol, and carvacrol) as novel solvents and diluents in the extraction process. By leveraging their unique hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) capabilities, we discuss both, the benefits and drawbacks, these terpenoids present in boosting selectivity in HAc separation. Our investigation provides insights into how the HBD and HBA characteristics of terpenoids influence the complexation in the organic phase, ultimately guiding the development of selective extraction systems.
Results:
As a first step, we evaluate terpenoids as pure and binary solvents (deep eutectic solvents) in terms of distribution coefficients and selectivity. Menthol was found to be the most promising alternative in comparison to conventional solvents. The high performance in terms of distribution coefficients and selectivity is attributed to its nature as HBA, while HAc acts mainly as an HBD. However, using terpenoids as constituents for deep eutectic solvents, undesired competition for binding sites of the DES forming molecules and the solute is observed, hindering the performance of DES in extraction of hydrophilic solutes such as HAc.
Based on the discussion of hydrogen bonding in extraction systems using terpenoids as pure or binary solvents, we explore their role as diluents in reactive extraction using TOPO and TOA. Our work focuses on the role of hydrogen bonding in stabilizing complexes in the organic phase, and we present insights into the underlying extraction mechanisms when using TOPO or TOA as extractants. We demonstrate why terpenoids have limited potential as diluents for TOPO. Instead, we show that in TOPO systems hydrophobic, non-polar diluents such as n-dodecane are favorable in countercurrent operation, since such diluents boost both, selectivity over water and extraction efficiency.
In contrast, terpenoids show great potential as diluents for TOA. Remarkably, with carvacrol as diluent and no electrolyte in the system, single-stage extraction efficiencies of close to 100% can be achieved at low pH without a molar excess of the extractant (see Figure 1). Furthermore, with Na2SO4 in the system, the selectivity toward HAc over Na2SO4 is significantly increased using terpenoids as diluent compared to conventional diluent/extractant systems like 1-decanol/TOA. Based on these findings, we show how strong asymmetric HBDs such as carvacrol and thymol contribute to TOA-complex stabilization. In summary, our results highlight the potential of terpenoid-based reactive extraction systems to enable efficient and selective recovery of HAc from gas fermentation processes.
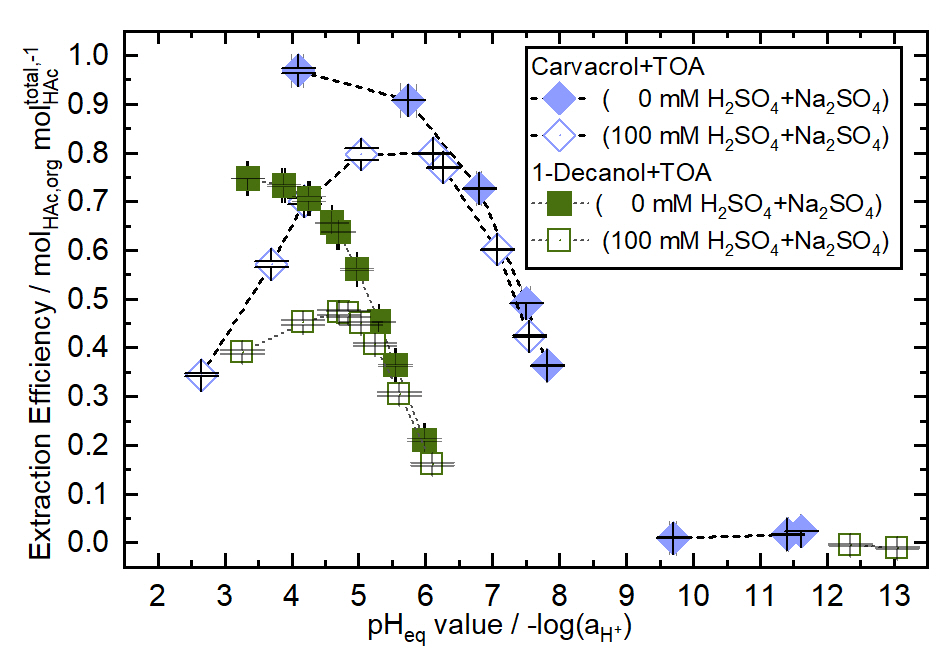
Figure 1: Extraction efficiency of acetic acid (HAc) as a function of pH using trioctylamine (TOA) dissolved in 1-decanol and carvacrol. The extraction was performed with a 1:1 stoichiometric ratio of TOA to HAc, at 25 °C, and equal mass of organic and aqueous phase (phase ratio 1:1).
- [1] M. Kircher, T. Schwarz, CO2 and CO as Feedstock, Springer International Publishing, Cham 2023.
- [2] X. Huang, Z. Li, Y. Tian, Chinese Journal of Chemical Engineering 2018, 26 (8), 1631 – 1643. DOI: https://doi.org/10.1016/j.cjche.2017.10.030
- [3] L. Sprakel, B. Schuur, Separation and Purification Technology 2019, 211, 935 – 957. DOI: https://doi.org/10.1016/j.seppur.2018.10.023
- [4] M. Gausmann, C. Kocks, J. Pastoors, J. Büchs, N. Wierckx, A. Jupke, ACS Sustainable Chem. Eng. 2021, 9 (28), 9336 – 9347. DOI: https://doi.org/10.1021/acssuschemeng.1c02194
- [5] M. Gausmann, R. Kiefel, A. Jupke, Chemical Engineering Research and Design 2023, 190, 590 – 604. DOI: https://doi.org/10.1016/j.cherd.2022.12.022
- [6] S. Tönjes, E. Uitterhaegen, K. de Winter, W. Soetaert, Separation and Purification Technology 2025, 356, 129881. DOI: https://doi.org/10.1016/j.seppur.2024.129881
- [7] C. B. Rasrendra, B. Girisuta, H. H. van de Bovenkamp, J. Winkelman, E. J. Leijenhorst, R. H. Venderbosch, M. Windt, D. Meier, H. J. Heeres, Chemical Engineering Journal 2011, 176-177, 244 – 252. DOI: https://doi.org/10.1016/j.cej.2011.08.082
- [8] M. Matsumoto, S. Uenoyama, T. Hano, M. Hirata, S. Miura, J. Chem. Technol. Biotechnol. 1996, 67 (3), 260 – 264. DOI: https://doi.org/10.1002/(SICI)1097-4660(199611)67:33.0.CO;2-Y
- Abstract category selection: