Oral Presentation International Solvent Extraction Conference 2025
Solvent Extraction as the Interface to Rare Earth Separation Refineries (122661)
The rare earths are a group of 15 elements (the lanthanides and yttrium) that are always encountered together in ore-bodies. While they are not rare, four of the rare earth elements (praseodymium, neodymium, terbium and dysprosium) have become increasingly critical in the shift towards electrification, with applications in electric vehicle motors and wind turbines. These four elements are currently responsible for greater than 90% of the contained value of a given deposit.
The rare earth magnet supply chain consists of several processing steps, namely:
- Mining;
- Beneficiation of the ore to generate a mineral concentrate;
- Processing of the mineral concentrate to a purified mixed rare earth intermediate by a hydrometallurgical process;
- Separation of the rare earths into separated rare earth oxides by solvent extraction;
- Metallisation of the rare earth oxides;
- Alloying of the rare earth metals;
- Production of rare earth magnets; and
- Production of the final product.
The rare earths are recovered from hard rock and placer deposits containing minerals such as monazite, xenotime and bastnaesite. One of the conventional processing routes is the so-called acid-bake flowsheet (Figure 1). After beneficiation, the mineral concentrate is baked at >200 ℃ with sulfuric acid, and then leached with water and purified by neutralisation and ion exchange to generate a purified rare earth sulfate solution.
The purified solution can be subjected to a carbonate precipitation step to generate a mixed rare earth carbonate (MREC), which is then redissolved with either HCl or HNO3 and separated by solvent extraction. This approach is typically used if the solvent extraction facility is not co-located with the mineral processing plant, as it allows for the rare earth intermediate to be transported between the facilities. However, there are several drawbacks to this approach, namely:
- The reagent cost associated with precipitating and then redissolving the rare earth carbonate, which is considerable;
- Addition of either sodium or ammonium into the barren sulfate solution, which must be managed at the hydrometallurgical plant; and
- Due to the presence of uranium in the ore, the MREC may contain the radioisotope actinium-227 at levels that result in the MREC being classified as radioactive material for transport purposes.
This talk discusses several different solvent extraction processes that can be used to replace the MREC precipitation step. The feed to the first solvent extraction process is the purified rare earth sulfate solution; and so this approach requires the refinery to be co-located with the hydrometallurgical facility. There are a number of different extractants and flowsheets that can and have been used, and selection of the preferred process is far from trivial. The potential advantages of this approach are:
- Reagent savings associated with not having to precipitate the rare earth carbonate;
- Potential Capex/Opex savings in the water treatment plant of the hydrometallurgical plant by not introducing sodium or ammonium into the SX raffinate;
- Rejection of actinium-227 in the solvent extraction process thus ensuring that the radioactivity is retained at the mineral processing facility;
- The possibility to reject low-value lanthanum/cerium to the SX raffinate if desired; and
- Realisation of more of the value of the deposit by further downstream processing.
This presentation will focus on the balance between the benefits of the various solvent extraction processes against the increase in complexity and capital cost compared to a relatively simple MREC precipitation circuit.
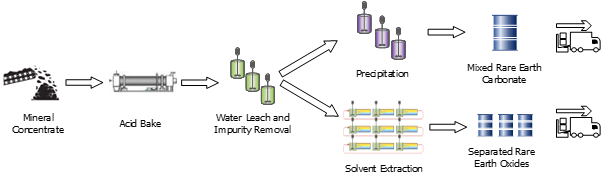
Figure 1: Comparison of MREC Precipitation and SX Approaches
- Abstract category selection: