Oral Presentation International Solvent Extraction Conference 2025
Contribution of extractant aggregate size to the selectivity of solvent extraction probed by SANS (120457)
Background: Solvent extraction efficiency, and more specifically the metal transfer from the aqueous phase to the organic phase, has so far been exclusively described through the complexation mechanism of the cation by the extractant molecules. However, recent finding has demonstrated a strong impact of the extractant aggregate structures on the process.[1, 2] It was thus observed that aggregates formed in the organic bulk phase contribute to metal size recognition impacting the selectivity, while the abundance of extractant/aggregates at the interface contribute to metal transfer in the organic phase impacting extraction kinetics.[3] In this way, this aggregation property has to be taken into account when designing new extraction system. However, finely adjust the structures formed in the organic phase remain a challenge as the factors influencing their formation, shape, and size are not clearly understood.
Methods: Neutron technologies, such as neutron reflectivity (NR) and small-angle neutron scattering (SANS), are ones of the most powerful tools to investigate the structures of extractant aggregates at the interface and in the organic bulk phase respectively. In addition, as the size recognition effect for the separation of Pd(II) and Nd(III) was initially observed using N,N’-dibutyl-N,N’-dimethyl-2-tetradecylmalonamide extractant (DBMA) in toluene and heptane,[4] it has been decided to investigate mixtures of these two solvents at different volume ratios. These mixtures were contacted to several aqueous solutions at different nitric acid and/or lithium nitrate concentrations. In this way, the role of solvent properties, proton (H+), and nitrates (NO3−) on the size recognition effect can be decorrelated. In this study, the extractant aggregate size in these toluene/heptane mixtures was determined using SANS at JRR-3 (Tokai, Japan), and correlated to Pd(II)/Nd(III) selectivity coefficients determined using ICP-OES.
Results: From the SANS data analysis (see Fig. 1a and b), three main factors influencing aggregate size have been pointed out: (i) the acidity of aqueous phase (i.e., [H+]aq), (ii) the extraction of nitrate ions (i.e., [NO3−]org), and (iii) the relative permittivity of the diluent (i.e., εR). In this way, bigger aggregates were obtained in heptane contacted to 5M HNO3 (RG = 4.44 nm), whereas the smallest ones were obtained in toluene contacted to 1M HNO3 (RG = 0.53 nm), with effects (i) and (ii) becoming more pronounced as the permittivity decreases (i.e. in pure heptane). In parallel, extracted water quantity (i.e. qwater) decreases when [H+]aq, or [NO3−]org increase or when εR decreases (see Fig. 1c), and has only shows a weak effect on the aggregate size. In this way, these factors can be classified according to their impact on the aggregation process, from strong to weak: εR > [H+]aq > [NO3−]org > qwater. Finally, Pd(II)/Nd(III) selectivity coefficients were shown to continuously decrease as the size of the extractant aggregate increases (see Fig. 1d).
Conclusion: In this study, the impact of several factors on extractant aggregate size in the organic phase has been investigated and directly correlated to the Pd(II)/Nd(III) selectivity coefficient which is maximum for small aggregates as they cannot accept the large Nd3+ ions in their hydrophilic core. In this way, it has been demonstrated that the selectivity can be finely tuned by adjusting either the organic phase composition (toluene content) or the aqueous phase composition (i.e. [HNO3]aq or [LiNO3]aq), and thus be used to adjust the solvent extraction efficiency. This brings a new insight in term of separation control that can help designing new separation steps and processes while using a unique extractant molecule. In a future work, external stimuli will be applied to tune the selectivity on unique chemical systems.
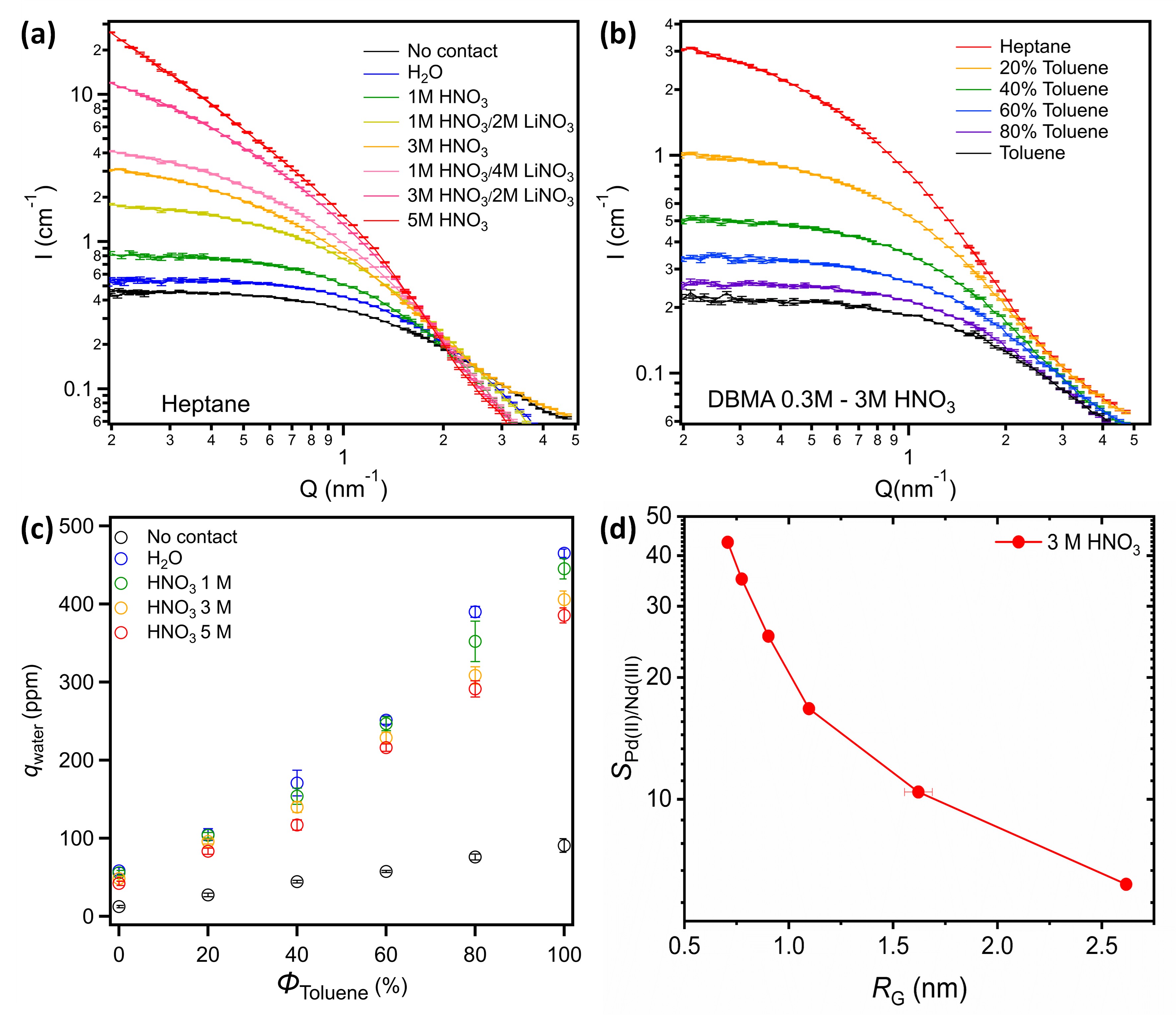
Fig. 1: Effect of aqueous phase composition (a) and organic composition (b) on SANS spectra, and impact of aggregate size variation on water extraction (c) and selectivity coefficient S (d).
- [1] J.-F. Dufrêche, T. Zemb, Chem. Phys. Lett., 622, 2015, 45–49 (DOI: 10.1016/j.cplett.2014.11.028)
- [2] J. Rey, M. Bley, J.-F. Dufrêche, S. Gourdin, S. pellet-Rostaing, T. Zemb, S. Dourdain, Langmuir, 33, 13168-13179, 2017 (DOI: 10.1021/acs.langmuir.7b02068)
- [3] C. Micheau, Y. Ueda, R. Motokawa, K. Akutsu-Suyama, N. L. Yamada, M. Yamada, S. A. Moussaoui, E. Makombe, D. Meyer, L. Berthon, D. Bourgeois, J. Mol. Liq., 401, 2024, 124372 (DOI: 10.1016/j.molliq.2024.124372)
- [4] R. Poirot, X. Le Goff, O. Diat, D. Bourgeois, D. Meyer, ChemPhysChem Commun., 17, 2016, 2112-2117 (DOI: 10.1002/cphc.201600305)
- Abstract category selection: