Poster Presentation International Solvent Extraction Conference 2025
Basic Research on the Application of Secondary Lithium Resource Recycling and Utilization (#102)
With the gradual depletion of fossil energy resources and growing global concerns regarding environmental protection, the exploration and development of new energy sources have become an international consensus. Lithium, as an indispensable material for electric vehicles and controlled nuclear reactors, has now emerged as a critical resource related to national energy security. The research team at the Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, has designed and developed dozens of extraction systems tailored to the characteristics of various lithium-containing solutions, including high magnesium-to-lithium ratio salt lakes (Qinghai), high sodium-to-lithium ratio salt lakes (South America), lithium precipitation mother liquors, and recycled solutions from spent lithium batteries. Concurrently, we have conducted comprehensive studies on related mechanisms and extraction processes.
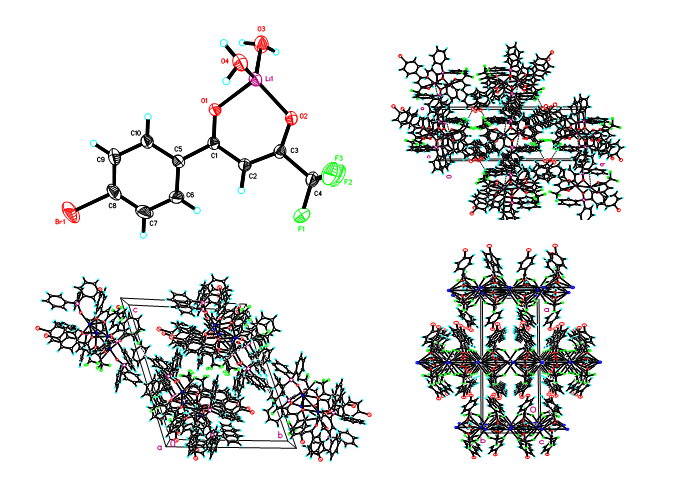
The research team employed equilibrium extraction methods to identify optimal synergistic extraction systems for different extraction systems and determined the corresponding optimal equilibrium extraction conditions for various feed solutions. In mechanistic investigations, analytical techniques including FT-IR, NMR, single-crystal diffraction, and computational chemistry were utilized to elucidate the structures of complexes formed during the extraction of lithium, sodium, potassium, and other metal ions. These analyses provided molecular-level insights into the extraction mechanisms of organophosphorus and diketone-based systems for alkali metal ions.
Building upon these foundational studies, process scale-up experiments were conducted using mixed-settler and centrifugal extractors with feed materials including brines from Da Qaidam, Cha'erhan, and Dong-Tai salt lakes in Qinghai, lithium precipitation mother liquors, lithium battery recycling tail liquors, and brine from Argentina's Diabillios salt lake. The process demonstrated stable operational data, yielding lithium-enriched solutions with an average lithium concentration of 28 g/L and total impurity content below 0.5 g/L, while achieving an overall lithium recovery rate exceeding 90%. Through comprehensive component analysis of equilibrium stage samples, the distribution patterns of lithium and other ions (Na⁺, K⁺, Ca²⁺, Mg²⁺, etc.) during separation, purification, and concentration processes were clarified. These findings provide scientific foundations for the efficient recovery of lithium from diverse lithium-containing brines.

- Abstract category selection: