Poster Presentation International Solvent Extraction Conference 2025
Viscosity and Density Measurements of Uranium-Loaded Monoamide Extractants (#104)
Uranium purification has been a topic for discussion since the mid-twentieth century, placing emphasis on the use of solvent extraction to recover uranium using tributyl phosphate (TBP) [1]. Since the outset of this process development, TBP has remained the primary contender for commercial use. Recently interest in alternative extractants has grown, namely in the class of monoamide extractants. These extractants provide the benefits of following the CHON (carbon, hydrogen, oxygen, and nitrogen) principle and can provide pathways for proliferation-resistant flowsheets [2, 3]. Due to widespread interest, research related to the more popular candidates is spread throughout different countries and research groups. This work aims to directly compare the most popular candidates with TBP under optimized conditions with standardized tests, eliminating discrepancies between studies. The extractants under review include TBP, N,N-di-2-ethylhexyl-isobutyramide (DEHiBA), N,N-di(2-ethylhexyl)butanamide (DEHBA), and N,N-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA), all diluted in an isoparaffinic hydrocarbon solvent, Isopar-L. For application in the nuclear industry, these extractants are tested under ideal process conditions (i.e., extractant concentrations frequently tested throughout other works) and with uranium as the target constituent for extraction [3–5].
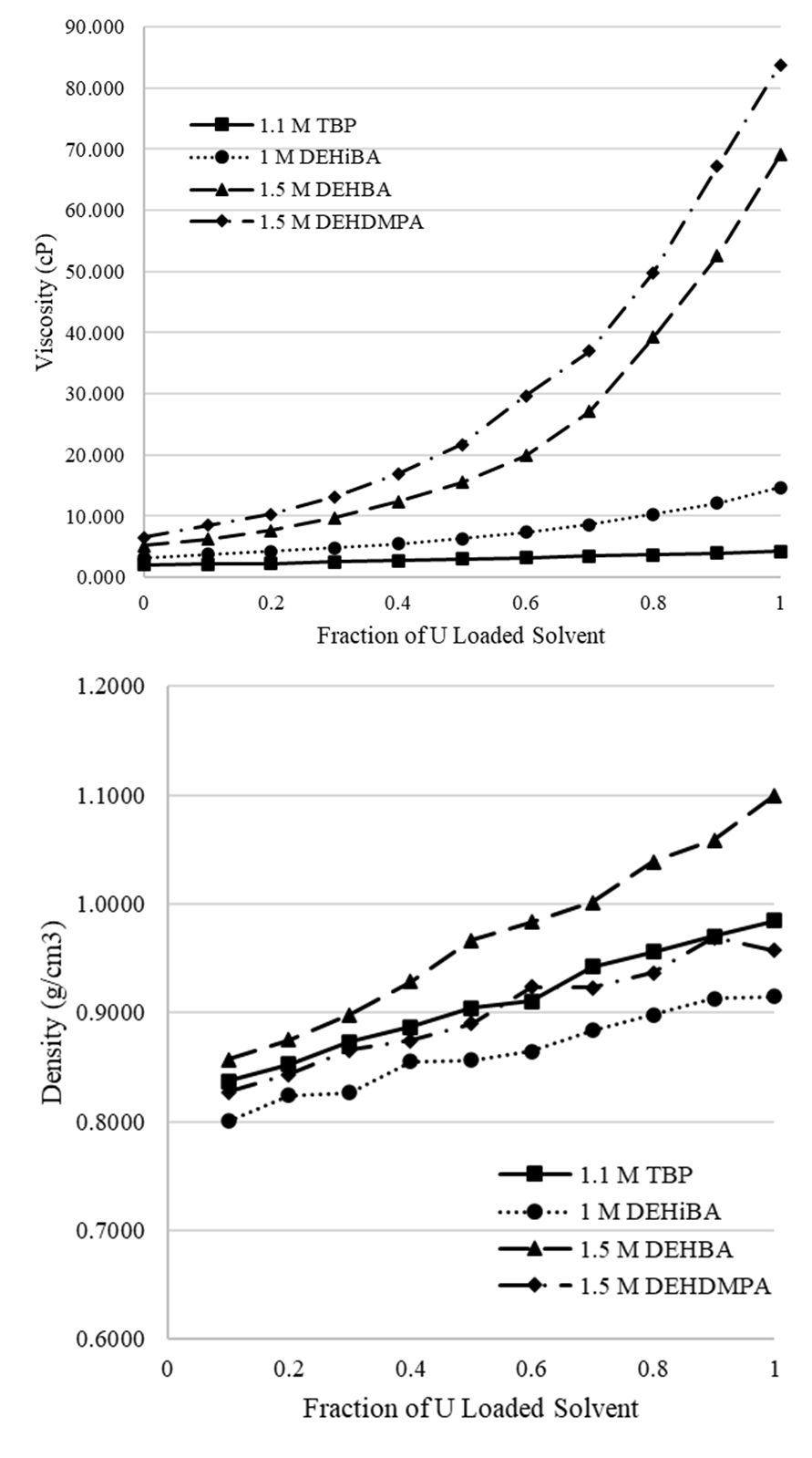
With consideration for compatibility in solvent extraction systems, the viscosity and density are important physical properties to quantify. At a more fundamental level, the premise of mass transfer itself in liquid-liquid extraction systems is a complex hydrodynamic process, and characterization of fundamental properties contributes to a more complete understanding [6] of this behavior. These properties also change as uranium is loaded into the organic phase, which has implications on process optimization, throughput, and equipment operation. Furthermore, systematic studies documenting the physical properties of solvent systems loaded with uranium are largely absent from the literature. For these reasons, the viscosities and densities of the selected monoamide extractants alongside that of TBP were measured with increasing uranium concentrations in the organic phase. This gradient of concentration was prepared with volumetric additions of the solvent solution to an aliquot of the solvent fully loaded with uranium. All measurements were taken at ambient temperature, measuring 21℃.
As shown in the attached figure, a clear trend is visible with the 1.5 M concentration extractants, DEHBA and DEHDMPA, with the viscosity increasing exponentially with increasing uranium loading. The 1 M DEHiBA also displayed this trend but to a much smaller degree and with lower overall viscosities. The 1.1 M TBP trend was linear. No non-Newtonian behavior was observed in the viscosity measurements of all samples. These trends also correlate well with a similar study [7]. Across all four extractants, the density showed a near linear increase, with a consistent ranking from lowest to highest of 1 M DEHiBA < 1.5 M DEHDMPA < 1.1 M TBP < 1.5 M DEHBA. These results are displayed in the attached figure and will be evaluated more closely with pending ICP-MS results.
For DEHBA and DEHDMPA specifically, the higher extractant concentration seemingly provides a significant increase in viscosity. However, at lower extractant concentrations the extraction efficiency is far below that of TBP. A comparison of equal extractant concentrations of 0.5 M with the percentage of extracted uranium from an identical aqueous feed provides the following results: DEHDMPA at 11%, DEHBA at 12%, DEHiBA at 14%, and TBP at 21%. Comparatively, at ideal extractant concentrations, uranium extraction from the same feed yields 1.5 M DEHDMPA at 37%, 1.5 M DEHBA at 44%, 1 M DEHiBA at 29%, and 1.1 M TBP at 39%. The increased extractant concentrations in the monoamides allow for an increased carrying capacity, but at the cost of hydrodynamic viability.
This presentation of data on the viscosity and density increase as a function of uranium loading alongside the extraction of uranium by each extractant provides insight into the challenges faced with exploring new extractant systems.
- Warf, J. C., Extraction of Ce(IV) Nitrate by Butyl Phosphate, AECD-2524, August 1947
- Paviet-Hartmann, P., Cerefice, G., Riveros Stacey, M., & Bakhtiar, S., Analysis of Nuclear Proliferation Resistance Reprocessing and Recycling Technologies, ICONE 19-43062, May 2011
- Miguirditchian, M., Chareyre, L., Sorel, C., Bisel, I., Baron, P., & Masson, M. (2008), Development of the GANEX Process for the Reprocessing of Gen IV Spent Nuclear Fuels, Atalante 2008: Nuclear fuel cycle for a sustainable future, France
- Authen, T. L., Adnet, J.-M., Bourg, S., Carrott, M., Ekberg, C., Galán, H., Geist, A., Guilbaud, P., Miguirditchian, M., Modolo, G., Rhodes, C., Wilden, A., & Taylor, R. (2022), An Overview of Solvent Extraction Processes Developed in Europe for Advanced Nuclear Fuel Recycling, Part 2—Homogeneous Recycling, Separation Science and Technology, 57:11, 1724–1744, DOI: 10.1080/01496395.2021.2001531
- Ban, Y., Hotoku, S., Tsubata, Y., & Morita, Y. (2014), Uranium and Plutonium Extraction from Nitric Acid by N,N-Di(2-Ethylhexyl)-2,2-Dimethylpropanamide (DEHDMPA) and N,N-Di(2-Ethylhexyl)Butanamide (DEHBA) using Mixer-Settler Extractors, Solvent Extraction and Ion Exchange, 32:4, 348–364, DOI:10.1080/07366299.2013.866850
- Rydberg, J., (Ed.), Solvent Extraction Principles and Practice, Revised and Expanded. United States: Taylor & Francis, 2004
- Pleines, M., Hahn, M., Duhamet, J., & Zemb, T. (2020), A Minimal Predictive Model for Better Formulations of Solvent Phases with Low Viscosity, EPJ Nuclear Sciences & Technologies, 6:3
- Abstract category selection: