Oral Presentation International Solvent Extraction Conference 2025
Optimizing flowsheet for selective recovery of cobalt, nickel, manganese, and lithium from black mass using Ionquest SX phosphinic, phosphonic, and phosphoric acids (121438)
The growing demand for valuable metals from challenging ores and the recycling of secondary sources, such as lithium-ion batteries, underscores the urgent need for innovative extraction methods. This study addresses this gap by optimizing a solvent extraction flowsheet using phosphinic acid, phosphonic acid, and phosphoric acid — specifically IONQUEST® 290, IONQUEST® 220, and IONQUEST® 801 — as extracting agents (SX) for the selective recovery of key metal ions: manganese (Mn), cobalt (Co), nickel (Ni), and lithium (Li) starting from black mass residue. The unique chemical properties, solubility, and interaction mechanisms of these acids with metal ions are analyzed to enhance extraction efficiency.
In this work, the optimized flowsheet will be shown based on a series of laboratory batch experiments were conducted to identify optimal operational parameters, and eventually validated through lab scale counter-current experiments, demonstrating the practical applicability of the proposed method.
The starting solution is a sulfuric acid pregnant leach solution (PLS) derived from black mass residue, containing the following metal concentrations: 8 g/L Co, 7 g/L Mn, 22 g/L Ni, and 3 g/L Li, along with impurities predominantly consisting of aluminum (Al), copper (Cu), and iron (Fe). Solvent extractants products - IONQUEST® 290, IONQUEST® 220, and IONQUEST® 801 provided by Italmatch Chemicals Spa – were diluted in ESCAID 110 provided by Exxon Mobil at concentration between 7 – 14 v/v%. pH adjustment and/or SX saponification was performed using NaOH 13 wt%. In stripping and scrubbing circuits, fresh sulfuric acid was used at different concentration.
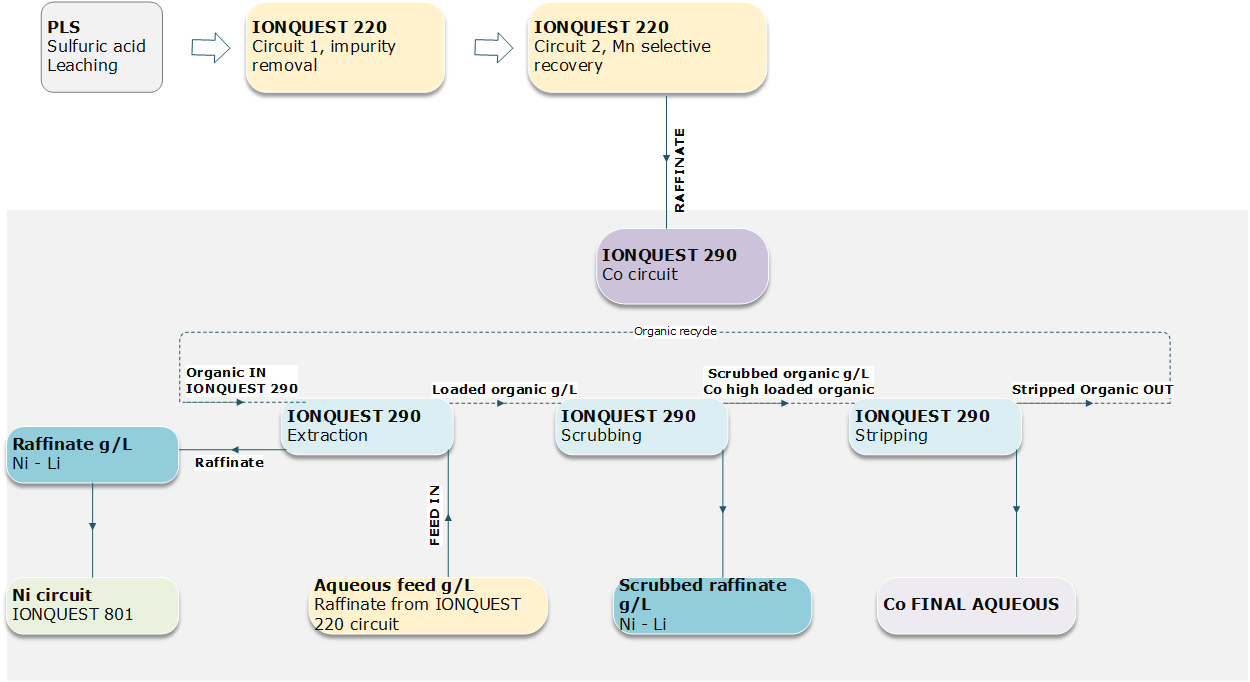
One of the fundamental steps of the overall solvent extraction flowsheet is the impurity removal steps prior the selective recovery of Co, Ni and Li. The removal of metal such as Fe, Al if present Ca and Mg may easily alter the final targeted purity.
Preliminary results indicate that phosphoric acid – SX (IONQUEST® 220) effectively removes impurities, allowing for a cleaner PLS for subsequent extraction stages. The study demonstrates that proper control of pH can significantly enhance the selective recovery of Al and Fe. After removing impurity, the raffinate can be recirculated in a specific circuit for Mn, where the loaded organic is then stripped using 2 mol/L H2SO4. The overall process achieved a Mn yield of about 90%.
Further optimization with phosphinic acid – SX (IONQUEST® 290) revealed enhanced selectivity for Co over Ni, particularly at adjusted pH levels, maximizing the recovery of both metals. The Co circuit consisted of 3 stages of extraction, 4 stages of scrubbing and 3 stages of stripping. A scrubbed raffinate reach in Ni can be obtained with 5 g/L of Co sulphate solution. Consequently, final Co raffinate resulted from stripping operations (1 mol/L H2SO4 used) showed 95 % of cobalt recovery.
The integration of phosphonic acid - SX (IONQUEST® 801) in the final extraction stages demonstrated a potential for differentiating between Ni and Li, facilitating a more efficient and selective recovery process. To minimize the loss of Li, Ni extraction is conducted with 7-10 v/v% of organic solvent with pH between 4.8 - 5.0. The work demonstrated that using lower concentration of extractant coupled with suitable O/A ratios can be a reasonable solution for Ni-Li separation challenge.
- Abstract category selection: