Poster Presentation International Solvent Extraction Conference 2025
Highly Selective Recovery of Platinum Group Metals from Spent Automotive Catalysts Using Extractant-Containing Hydrophobic Deep Eutectic Solvents (#106)
Introduction
Platinum group metals (PGMs) are indispensable in various industrial applications due to their exceptional properties. Notably, platinum, palladium, and rhodium serve as key components in automotive catalysts, enabling the purification of exhaust gases from car engines. However, PGMs are unevenly distributed, and their reserves are finite. Thus, developing an efficient recycling process for recovering these metals from spent automotive catalysts is crucial.
In conventional hydrometallurgical recycling processes for PGMs, high concentrations of inorganic acids are used to dissolve various metals, followed by the selective recovery of PGMs through solvent extraction with organic solvents. However, this approach poses significant environmental concerns, particularly due to the generation of acidic effluents and the extensive use of volatile and toxic organic solvents.
Recently, greener alternatives such as ionic liquids and deep eutectic solvents (DESs) have been explored for the selective dissolution of PGMs from spent automotive catalysts. DESs are mixtures of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) that form a liquid at room temperature. As environmentally friendly solvents, they hold promise as substitutes for strong inorganic acids in recycling processes.
We developed a hydrophobic DES with enhanced hydrophobicity and metal selectivity by incorporating an extractant and a hydrophobic component. The selective dissolution of PGMs from spent automotive catalysts was investigated using this hydrophobic DES in combination with additives that promote PGM leaching. Furthermore, PGM recovery into the aqueous phase was attempted by contacting the metal-loaded hydrophobic DES with an appropriate aqueous solution. Additionally, the recyclability of the hydrophobic DES was evaluated.
Results
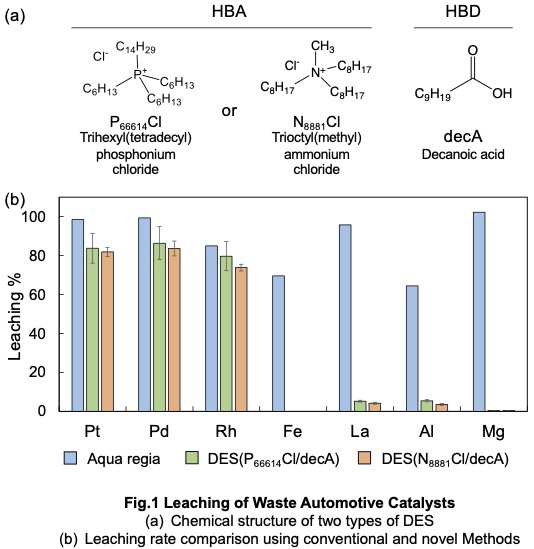
Two types of DES were prepared using trioctylmethylammonium chloride (N8881Cl) or trihexyltetradecylphosphonium chloride (P66614Cl) as the HBA and decanoic acid as the HBD. The chemical structures of the DES components are shown in Fig. 1(a). The leaching results of spent automotive catalysts using conventional inorganic acids and the two DES are presented in Fig. 1(b). While highly concentrated inorganic acids achieved efficient PGM leaching, they also caused extensive dissolution of Al, La, and Mg. In contrast, DES with additives enabled the selective dissolution of PGMs while significantly suppressing the leaching of Al, La, Mg, and other metals. Two possible explanations have been considerd. First, the use of a hydrophobic solvent instead of an aqueous solution may contribute to the observed selectivity. Metals with high charge densities, which readily form complexes with water, tend to leach in aqueous solutions. However, since hydrophobic DES contains only trace amounts of water, it inhibits the leaching of metals with high charge densities, such as La, Al, Ba, and Mg. Second, the metal-coordinating properties of N8881Cl and P66614Cl in DES may also play a role in selective leaching. The metal-coordination ability of DES is crucial for effective leaching. P66614Cl and N8881Cl, key components of DES, act as efficient extraction agents for PGMs due to their strong affinity for these metals, enabling coordination and stability within the hydrophobic DES after leaching.
Next, metal separation from DES into aqueous solutions was examined. The stripping process involved sequential contact between DES and four aqueous solutions: water, 5 M ammonium chloride, 0.005 M thiourea, and 10 M ammonium nitrate. In the first stripping stage, nearly all unwanted metals, including Al and La, were effectively removed, though approximately 10% of Rh was also extracted. In the second stage, Rh was selectively recovered, followed by Pd in the third stage and Pt in the fourth stage, each with high purity in their respective aqueous solutions.
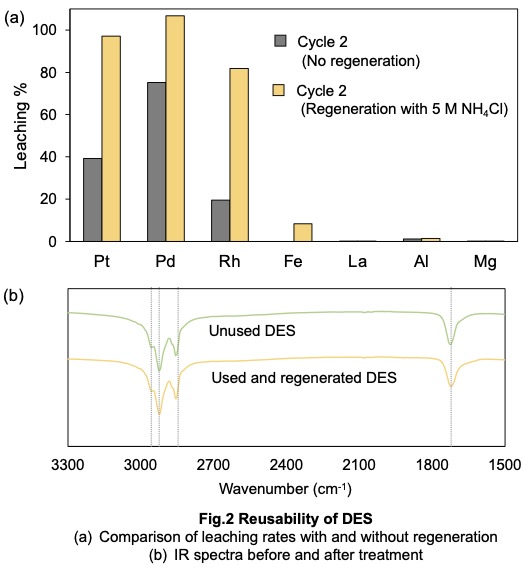
The results of the study on the repeated use of DES are presented in Fig. 2(a). Hydrophobic DES was reused after metal removal. When DES was used without regeneration, the suppression of unwanted metals was maintained, but the PGM leaching rate decreased. In contrast, when DES underwent regeneration with 5 M ammonium chloride, the PGM leaching rate remained consistent with that of the initial leaching. Fig. 2(b) presents the IR spectra of hydrophobic DES before use and after use with regeneration, clearly showing no significant change in the solvent composition. These findings confirm that DES can be effectively reused through the regeneration process.
- Abstract category selection: