Poster Presentation International Solvent Extraction Conference 2025
Acid mist suppressant: a non-fluoro environmentally friendly surfactant to reduce acid mist formation in copper electrowinning (#113)
ABSTRACT: Acid mist formation is a significant safety concern during electrowinning operations and there is significant interest in finding new solutions to this problem due to the discontinuation of an industry standard fluorine-based additive. This study discloses that a non-fluorine-based additive can be utilized as an environmentally friendly acid mist suppressant, low-foaming with no detrimental effect on the cathode quality and solvent extraction. The effect of these additives was laboratory assessed in comparison to the historically used fluorine-based additive and an existing natural extract-based surfactant. Initial results have shown that these additives not only effectively suppress the acid mist formation in electrowinning process but also improve cathode quality with no adverse effects on solvent extraction observed during laboratory trials.
KEYWORDS
Acid Mist Suppression, Electrowinning, Electrodeposition, Copper, Metal Extraction, Surfactant, Fluorine Free, Cathode Quality
1.1 Introduction
Sulphuric acid mist is generated during the final hydrometallurgical electrowinning stage of metal refining, viz. copper, nickel and zinc. Copper electrowinning is an essential process in the extraction of copper, involving the electrochemical deposition of copper from a rich copper sulphuric acid solution onto a cathode [1]. This mist is caused by the oxygen evolution at the anode, which forms as small bubbles that rise to the surface of the electrolyte, burst at the liquid/air interface [1][2]. These acid droplets are airborne and hazardous to the working environment for operators and causes excessive corrosion to the tank-house structure and ancillary equipment [1][2].
Conventionally, the industry has used a combination of physical barriers alongside chemical additives to suppress acid mist formation. A fluorine-based additive has been widely used to reduce acid mist effectively [1]. However, due to the environmental persistence and potential health hazards associated with this class of surfactant, this additive has been discontinued. With this, the search for nonfluorine-based additives that can effectively suppress acid mist to an acceptable level has become an industry need.
1.2 Results
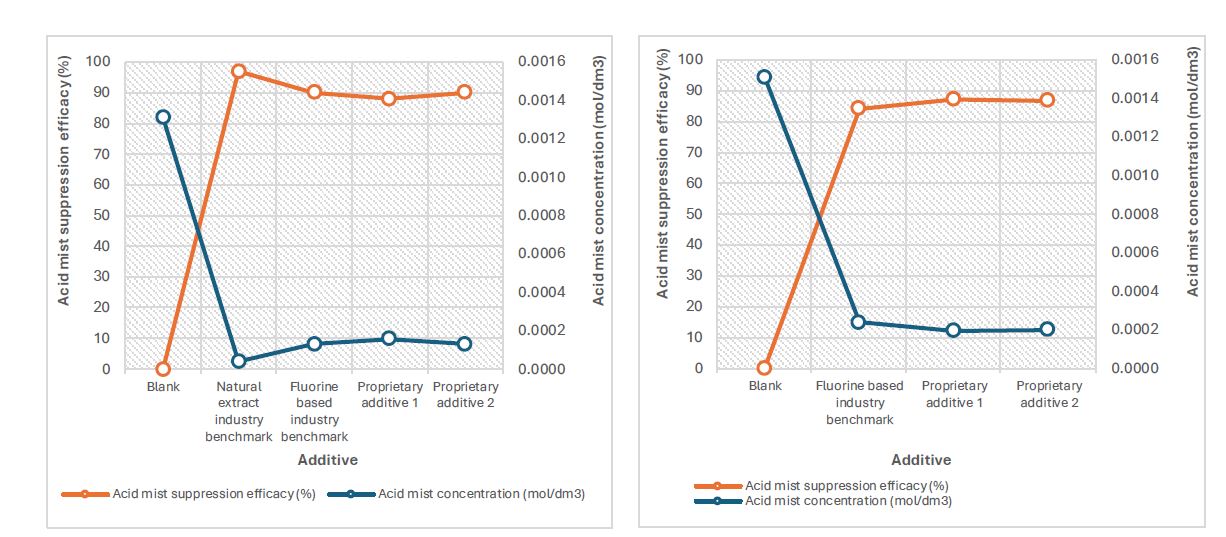
Testing was carried out for a blank (no surfactant), two industry benchmarks: a fluorinated product and a natural extract-based product, and two non-fluorine based proprietary additives at a dosage level of 20 ppm as shown in Table 1. For each experiment the acid mist suppression, Cu % recovery, and foam generation were recorded as shown in Table 1 and Figs. 1–2.
Table 1 Test results showing the acid mist suppression, Cu % recovery and foam generation for various additives (including two industry benchmarks) after 4 hours in an electrowinning cell.
|
Additive |
Active surfactant (ppm) |
500 A/m2 |
300 A/m2 |
||
|
Acid mist suppression (%) |
Acid mist suppression (%) |
Cu recovery (%) |
Foam generation |
||
|
Blank |
0 |
0 |
0 |
97.0 |
None |
|
Proprietary additive 1* |
20 |
87 |
88 |
99.8 |
None |
|
Proprietary additive 2* |
20 |
87 |
90 |
99.0 |
Moderate |
|
Fluorine based industry benchmark |
20 |
84 |
90 |
99.0 |
None |
|
Natural extract industry benchmark |
20 |
- |
97 |
96.3 |
High |
*These two additives are proprietary information and therefore the chemistry is not disclosed here – patent pending
|
Fig. 1 Acid mist suppression efficacy of two proprietary additives vs industry benchmarks at a current density of 300 A/m2 |
Fig. 2 Acid mist suppression efficacy of two proprietary additives vs industry benchmarks at a current density of 500 A/m2 |
1.3 Conclusions
- Two additives of proprietary technology have been tested on an electrowinning cell and have been found to have comparable performance to a proven fluorine-based industry benchmark with acid mist reduction values of 88-90% at a current density of 300 A/m2 and 87% at 500 A/m2.
- Proprietary additive 1 and the fluorine-based industry benchmark did not foam during testing at 300 A/m2.
- The two proprietary additives were found to produce excellent Cu recovery % values of 99.8% and 99.0% compared to the fluorine-based industry benchmark (99.0%).
- Proprietary additive 1 was found to produce an excellent appearance on the surface of the cathode.
References
[1] Roa T (2022) An investigation of acid mist formation and suppression mechanisms in copper EW plants. Master’s Thesis, University of Arizona
[2] Al Shakarji R (2012) Mechanisms of acid mist formation in electrowinning. PhD Thesis, James Cook University
- Abstract category selection: