Oral Presentation International Solvent Extraction Conference 2025
Towards characterization of retained ruthenium in the PUREX process (121045)
Liquid-liquid extraction is a fundamental separation technique in the processing of spent nuclear fuel by the PUREX (Plutonium-Uranium Reduction EXtraction) process. In this process, uranium and plutonium are separated using tri-n-butyl phosphate (TBP) diluted in aliphatic diluent. Ruthenium, contributes to beta-gamma emissions and makes up to 7 to 9% of the total mass of fission products.1 Its co-extraction, although limited, is problematic posing a challenge in the treatment process. Effective separation of the major actinides from Ru is therefore essential to improve the efficiency and selectivity of the process.
While process schemes are traditionally based on equilibrium data, mass transfer kinetics are typically studied and optimized afterwards. However, not all metals are extracted through the same mechanisms, hence different extraction rates have been observed in the literature.2 Consequently, the development of optimal liquid-liquid extraction processes requires a deep understanding of both the thermodynamic performance and the transfer kinetics of the extraction system.2 Based on the previous studies regarding ruthenium,3 our objective, is to study the influence of extraction mechanisms and mass transfer kinetics on the separation of major actinides from ruthenium in spent nuclear fuel using tributylphosphate extractant.
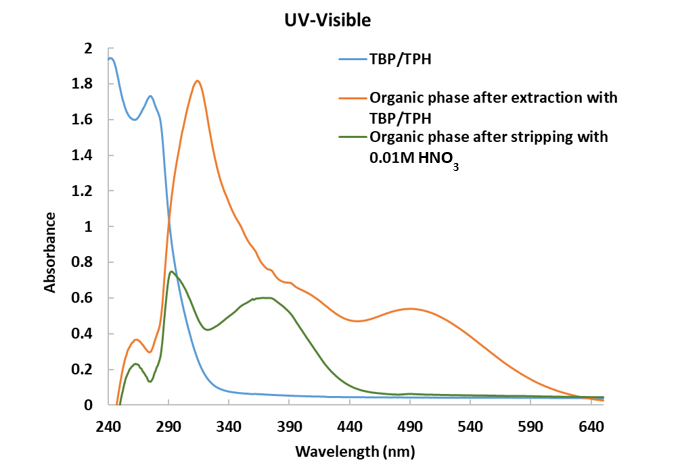
First experimental results using 30% TBP in Hydrogenated Tetrapropylen (TPH) showed a systematic retention of approximately 7% to 9% of the total ruthenium in organic phase after extraction from nitric acid. UV-Vis spectroscopy of the organic phase indicated the presence of a broad absorption band at 370 nm, even after successive stripping stages, confirming the presence of ruthenium in the organic phase, (Fig-1).
Figure 1: UV-Visible spectra of ruthenium in the organic phase after extraction and stripping
The low value of the ruthenium distribution ratio (ca. 0.10) is not consistent with stripping issues. We hypothesized the presence of at least two different Ru species in the organic phase: one major species that is readily stripped, while another minor species remains in the organic phase and resists stripping. These findings are consistent with previous studies,4,5 where the existence of multiple ruthenium species with varying extraction and back-extraction behaviors was suspected, further reinforcing the complexity of Ru speciation and its impact on phase transfer dynamics.
Our study has been dedicated to the complete description of Ru distribution between both phases, along with its speciation in the organic phase. A new methodology using X-ray fluorescence (XRF) has been developed for Ru quantification in organic phases to determine accurate distribution ratio. The complete characterization of the retained Ru complex is underway using various spectroscopy techniques.
The determination of the global transfer constants from aqueous to organic phase of different Ru species has been carried out using the single drop technique, in order to fully describe the Ru extraction and stripping mechanisms using TBP.
The findings from these studies will provide valuable insights into the relationship between mass transfer kinetics, the ion/extractant system, and the molecular phenomena occurring in each phase during the transfer process. This deeper understanding is essential for advancing liquid-liquid extraction processes and optimizing the handling of ruthenium in the spent fuel cycle, ultimately improving the efficiency and selectivity of nuclear fuel reprocessing.
- Radiochemistry and nuclear chemistry 3rd edition G.Choppin et al. Butterworth-Heinemann (2010) 593
- Moussaoui, Sayed Ali. Liquid/liquid separation governed by kinetics. Phd thesis, Montpellier University, 2021.
- Reddy, S. Ramakrishna, et al. "Extraction kinetics of ruthenium in the mixture of tri-n-butyl phosphate and n-dodecane." Progress in Nuclear Energy 86 (2016) 50-62.
- Maya, L. "Chemistry of extractable nitrosyl ruthenium species in the system nitric acid-tributyl phosphate-dodecane." Journal of Inorganic and Nuclear Chemistry 43.2 (1981) 385-390.
- Fletcher, J. M., C. E. Lyon, and A. G. Wain. "The partition coefficients of nitratonitrosylruthenium complexes between nitric acid and TBP phases." Journal of Inorganic and Nuclear Chemistry 27.8 (1965) 1841-1851.
- Abstract category selection: