Oral Presentation International Solvent Extraction Conference 2025
Machine learning-assisted development of extracting solvents with high Au(III) extractability and low aqueous solubility (121429)
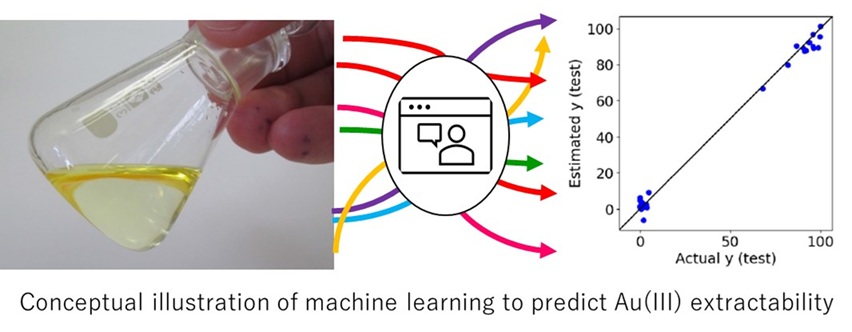
Tetrachloroauric acid (HAuCl4, Au(III)) is extracted by oxygen-containing solvents such as ethers and ketones, which have been applied worldwide in refining processes for precious metals. The aliphatic triether dibutylcarbitol (DBC) is the most popular solvent for Au(III) extraction. Au(III) is rapidly extracted by DBC with highly extraction capacity and selectively from other metals. Results of the Au(III) extractability by a series of aliphatic ketones and ethers suggests that solvents with relatively high polarity show high extractability. However, the extracting solvents should also have low water solubility to suppress leakage into the aqueous phase. In order to find suitable extracting solvents that have the contradictory properties of high Au(III) extractability and low aqueous solubility, machine learning models were constructed using the two values as objective variables.
The extraction percentage was used as the objective variable in machine learning. The aqueous solubilities of various organic solvents were obtained using HMDB (Human Metabolome DataBase), and their logarithmic values (logS) were used as another objective variable. The molecular structures of the solvents were expressed in simplified molecular-input line-entry system (SMILES) notation, and 208 descriptors of the solvents were obtained using RDKit and were used as explanatory variables. Machine learning was performed on a machine learning cloud service (Datachemical LAB, Tokyo, Japan). The dataset was divided to training and testing data in a ratio of 7:3 using the Kennard-Stone algorithm. Autoscaling was performed with a mean of 0 and a standard deviation of 1 for all descriptors, to align the scales of the descriptors. Using the training data, 25 types of models were constructed between the objective variables and descriptors. Each model was validated using the test data. To evaluate the predictive accuracy of the constructed model, the squared correlation coefficient (R2) , the root mean square error (RMSE), and the mean absolute error (MAE) were calculated.
In the comparison of 25 models, NGPR3 (Gaussian process regression) showed the highest accuracy in predicting the Au(III) extractability: The R2 value for the test data was more than 0.99. The small difference in predictions between the training data and the test data suggests the construction of a model with highly accuracy. Data division using the Kennard-Stone algorithm should contribute to the construction of the highly accurate model. The experimental data on the Au(III) extractability using 108 types of solvents in this study must be the largest compared to previous studies, and the machine learning model developed from the data is useful to predict Au(III) extractability using various organic solvents.
- T. Oshima, T. Koyama, N. Otsuki, Solvent Extr. Ion Exch. 39, 477-490 (2021).
- T. Oshima, K. Matsuzaki, A. Inada, K. Ohe, Sep. Purif. Technol. 258, 118008 (2021).
- T. Oshima, K. Miyake, AIChE J. 67 e17214 (2021).
- T. Oshima, Y. Iwakiri, A. Inada, Hydrometallurgy 220, 106106 (2023).
- Abstract category selection: