Oral Presentation International Solvent Extraction Conference 2025
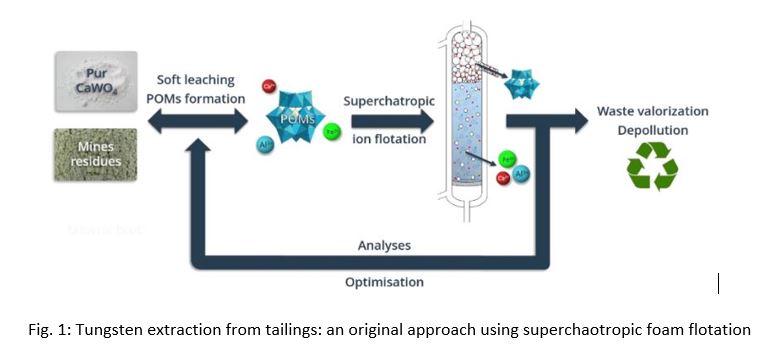
Green process for selective extraction: superchaotropic foam flotation of tungsten from mild conditions leaches of scheelite rich ores. (121554)
In order to provide a sustainable method of handling tungsten-rich wastes in the form of Scheelite, this study investigates the requirements for tungsten (W) element extraction from mining leftovers (CaWO4). These wastes indeed pose a major threat to the ecosystem.
Numerous studies that we have published demonstrate that ions of nanometric size (nano-ions) with low charge densities, like anionic boron clusters or certain polyoxometalates (POMs), strongly adsorb to neutral hydrated surfaces. The "superchaotropicity" of these specific nano-ions allows us to imagine and develop a number of separation science applications [3].
In 2023, we especially looked into utilizing foams made with a commercial non-ionic polyethoxylated surfactant to extract superchaotropic isopolyoxomolybdates, which are created by varying the pH and content of molybdate aqueous solutions. The [Mo36O112]8-, often referred to as the Krebbs anion with a low charge density, is the major species of the molybdate in solution at pH 1, where the flotation recovery of molybdenum species, using neutral foaming agents, is high and maximal. Since isopolyoxotungstate species produced in similar and acidic conditions are not superchaotropic, it has also been shown that molybdate may be separated from tungstate at pH 2, under similar acidic conditions, by superchaotropic ion flotation (see figure 1) [4].
Utilizing this specific superchaotropic interaction, we developed a proof of concept for the selective extraction of nano-ions using ion flotation in conjunction with a non-ionic foaming agent. This process was entitled: superchaotropic flotation. Then, as explained in this presentation, we show how effectively scheelite residues can be dissolved to produce phosphotungtate POM under mild conditions [1,2] at low concentrations of phosphoric and sulfuric acids.
The decomposition of scheelite in the synergistic H2SO4-H3PO4 mixture is indeed an effective method for extracting tungsten from mining concentrates. The driving force behind the dissolution of scheelite in the H2SO4-H3PO4 mixture is the formation of a soluble Keggin-type polyoxotungstate, [PW12O40]3-, that prevents the formation of very low solubility tungstic acid.
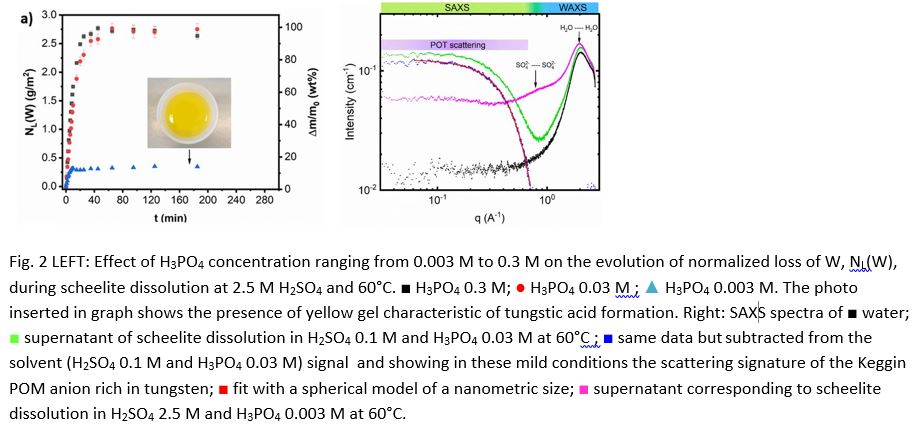
A multiparametric study of scheelite dissolution kinetics was carried out in the synergistic acid mixture (see figure 2). Specifically, the independent effects of temperature, acid concentration, and W:P molar ratio on the yield of [PW12O40]3- formation and the kinetics of scheelite dissolution were assessed. To achieve this, small-angle X-ray scattering (SAXS) technique was carried out in order to identify and quantify [PW12O40]3- under various operating conditions (see figure 3).
We can better constrain the stability range of [PW12O40]3- in terms of H2SO4 concentration and stoichiometric W:P ratio thanks to the results obtained and choose optimal and soft-leaching conditions that guarantee quick scheelite dissolution without causing surface passivation through secondary phase precipitation. In the presence of phosphoric acid, scheelite dissolved more slowly but remained effective at H2SO4 concentrations as low as 0.1 M. Even at low acidity levels, the effects of saturation phenomena on the dissolution kinetics were avoided. Actually, in this range of acidic conditions and at 60°C, anhydrite production is either negligible or occurs after the complete dissolution of scheelite [5].
Then we have shown that an effective extraction of tungsten in the form of the phosphotungstate [PW12O40]3- with pH-dependent selectivity and a yield close to 100% through superchaotropic ionic flotation from the leaching generated is achievable. The separation of superchaotropic species from other ions in solution—in this case, primarily calcium—was confirmed by the application of a non-ionic polyethoxylated surfactant, which encouraged the production of stable foams enriched in superchaotropic nano-ions.
The robustness of polyoxotungstate production under these acidic settings suggests a method that can resist variation in low acid concentrations and is still appropriate for mild leaching conditions, both of which being critical for the development of sustainable ore processing. The possibility of using superchaotropic ion flotation to separate and extract W from acidic leachates in the presence of high iron content, which is frequently present in ores, is further explored.
- Cao, C. et al., 2020. Study on leaching behaviour of tungstates in acid solution containing phosphoric acid. Hydrometallurgy, 197: 105392. Chen, X., Guo, F., Chen, Q., Liu, X. and Zhao, Z., 2019.
- Gürmen, S., Tımur, S., Arslan, C. and Duman, I., 1999. Acidic leaching of scheelite concentrate and production of hetero-poly-tungstate salt. Hydrometallurgy, 51(2): 227-238.
- Naskar, B., Diat, O., Nardello-Rataj, V. and Bauduin, P., 2015. Nanometer-Size Polyoxometalate Anions Adsorb Strongly on Neutral Soft Surfaces. The Journal of Physical Chemistry C, 119(36): 20985-20992.
- Skorzewska, K., Jonchère, A., Pasquier, C., Girard, L. and Bauduin, P., 2023. Superchaotropic ion flotation: A new concept for the extraction and separation of nanometer-sized ions by non-ionic surfactant-based foams. Separation and Purification Technology, 323: 124284.
- Legrand, V., Szenknect, S.,Diat, O., Girard, L. and Bauduin, P. , 2025. Dissolution of scheelite under soft acidic conditions via the formation of polyoxotungstate: kinetics and mechanism. Hydrometallurgy 21th march submitted.
- Abstract category selection: