Oral Presentation International Solvent Extraction Conference 2025
Speciation of Nitric Acid in Organic Phase: A Molecular Dynamics Study (121686)
In spent nuclear fuel reprocessing, solvent extraction is used to separate uranium and plutonium from fission products. After the fuel is dissolved in a nitric acid solution, the aqueous phase is contacted with an organic phase containing extractant molecules. This process transfers actinides to the organic phase but also causes the co-extraction of nitric acid. This phenomenon is relevant in the case of solvent extraction using monoamides as extractant molecules. At the molecular level, a number of questions remain unanswered: in what form is the acid extracted and what is its impact on speciation in the medium? A detailed understanding of this speciation is essential not only for understanding the underlying chemistry, but also for developing reliable thermodynamic models and improving chemical processes development. However, apart from studies into the speciation of metals in the presence of acid,1 there has been little research into the characterization of nitric acid species in the organic phase.2 It is due to the experimental limitations and difficulties in analyzing weak interactions in these organic media. In this work, we undertook molecular dynamics modelling of organic phases containing monoamide extractants in the presence of acid. The explicit integration of nitric acid into these simulations, a non-trivial challenge, required suitable assumptions to ensure the relevance of the results. The analyses of simulations reveal that nitric acid plays a key role in the speciation of the organic phase. The molecules of nitric acid establish hydrogen bonds with the extractant molecules and, with the few co-extracted water molecules, form well-defined species whose abundance depends on the organic acid concentration. Compared with previous studies, our molecular dynamics approach coupled to experimental data enable us to establish a more robust speciation of nitric acid. This work also improves the understanding of the physico-chemical equilibria governing these media. Finally, there is still a challenge: correctly incorporating nitric acid in the presence of plutonium(IV) into the molecular dynamics simulations.
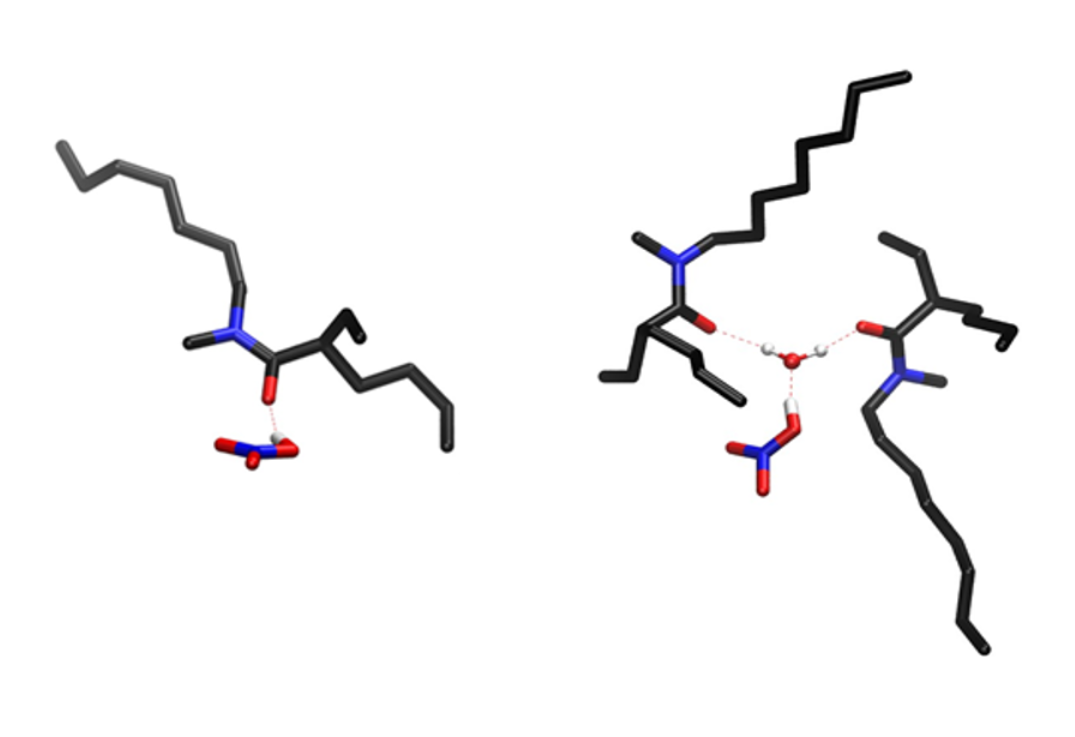
Figure 1. Molecular representation of two species appearing at high acidity in the organic phase containing MOEHA molecules (monoamide extractant). The aliphatic hydrogen atoms are not shown for clarity.
- (1) Acher, E.; Dumas, T.; Tamain, C.; Boubals, N.; Solari, P. L.; Guillaumont, D. Inner to Outer-Sphere Coordination of Plutonium( IV ) with N,N-Dialkyl Amide: Influence of Nitric Acid. Dalton Trans. 2017, 46 (12), 3812–3815. https://doi.org/10.1039/C7DT00031F.
- (2) Hall, G. B.; Campbell, E. L.; Bessen, N. P.; Graham, T. R.; Cho, H.; RisenHuber, M.; Heller, F. D.; Lumetta, G. J. Extraction of Nitric Acid and Uranium with DEHiBA under High Loading Conditions. Inorg. Chem. 2023, 62 (17), 6711–6721. https://doi.org/10.1021/acs.inorgchem.3c00288.
- Abstract category selection: