Oral Presentation International Solvent Extraction Conference 2025
Cs-137 extraction from chloride brines using calixarene crown ethers – selectivity explained by coordination chemistry (121761)
Cs-137 is an important fission product of uranium and plutonium, posing environmental risks due to its beta radiation and chemical similarity to biologically essential ions like sodium and potassium. Its accidental release, such as during the Fukushima-daichi incident, and its presence in nuclear waste repositories make its removal from contaminated environments crucial. However, Cs-137 recovery is challenging due to interference from high concentrations of sodium and potassium. Calix[4]arene 18-crown-6 ethers like calix[4]arene-bis(t-octylbenzo-crown-6) (BOBCalix) and 1,3-alt-25,27-bis(3,7- dimethyloctyl-1-oxy)calix[4]arenebenzocrown-6 (MAXCalix) have shown potential for selective extraction of cesium from nitrate media recently.[1-5] Recent studies indicate that MAXCalix may be effective in extracting Cs+ from chloride-rich solutions, making it a viable option for environmental decontamination.[6-7]
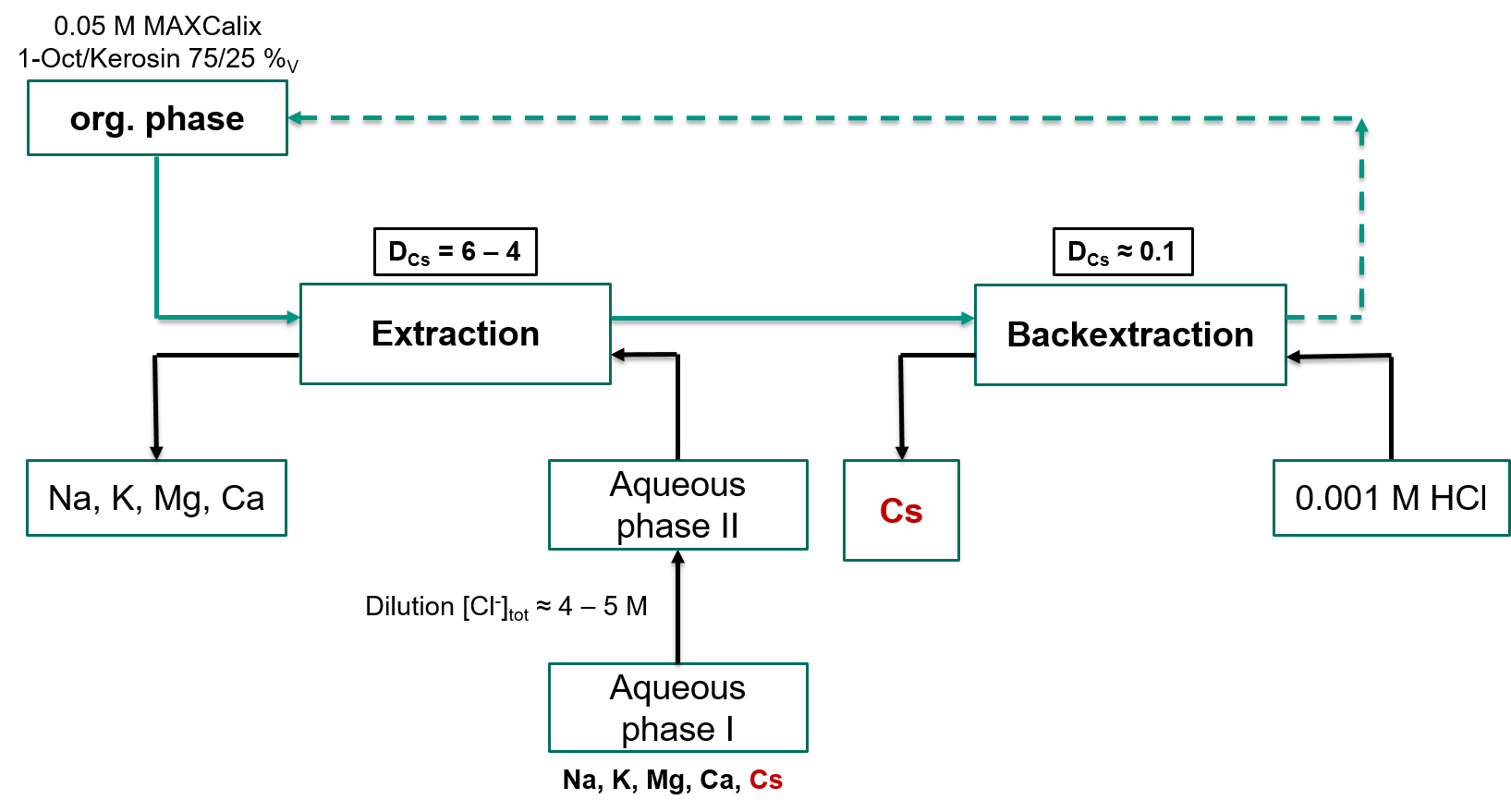
In this work, we propose a procedure for efficiently separating radioactive Cs+ from brine solutions on the laboratory scale by using solvent extraction. A solution of 50 mmol/L MAXCalix in kerosene/octanol 25/75 %v extracts Cs+ at chloride concentrations exceeding 0.3 mol L-1. The efficiency of extraction notably improves at higher chloride concentrations, resulting in Cs+ distribution ratios ranging between 4 and 6 at [Cl-] = 4.45 mol L-1, depending on the composition of the aqueous phase. Cs+ back-extraction is feasible into dilute hydrochloric acid. The system is sensitive to the presence of K+, but nevertheless features substantial tolerance margins against other alkali or earth alkali cations. This is important since the composition of the feed solution may vary strongly for different systems. Separation factors determined from mixed brine solutions containing Na+, K+, Ca2+, and Mg2+ range between 104 and 100, emphasizing the strengths of the developed procedure.
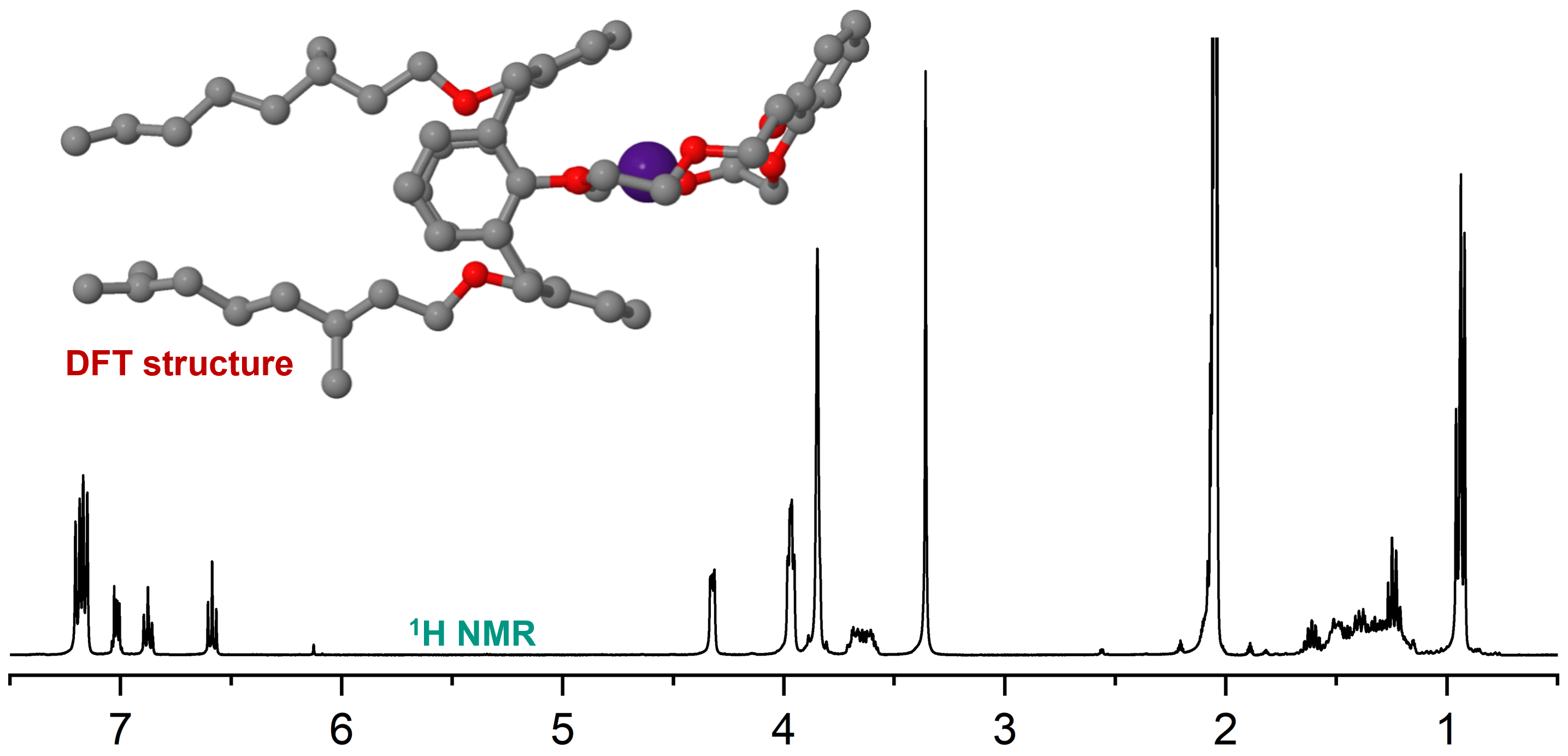
The extraction results may seem counterintuitive given the well-established 18-crown-6 ether chemistry, but highlights a gap in understanding the coordination chemistry of these ligands with chemically related ions. Therefore, we complemented the extraction studies with systematic investigations of the coordination chemistry of MAXCalix with M+ and M2+ ion from the alkali and alkaline earth metal series. NMR spectroscopy studies reveal that while MAXCalix efficiently coordinates large ions such as Cs+, it also forms complexes with smaller ions like Na+, highlighting the ligand’s versatility. The size of the ion directly influences the complex structure, with two distinct structural subtypes identified via NMR and DFT calculations. Additionally, π-interactions between the cation and the cation-facing benzene rings of the calix[4]arene backbone play an important role in stabilizing the complex. Larger ions like Cs+ benefit from π-interactions with both cation-facing rings, whereas smaller ions like K+ interact with only one ring, if any. These π-interactions are primarily drivers of the enhanced affinity for Cs+ and the resulting higher complex stability. Competitive NMR studies further confirm that the complex stability increases with increasing ionic radius, and ions of similar size show comparable stability of their complexes. These trends derived from the NMR studies are in excellent agreement with the high selectivity for Cs+ observed in the extraction studies.
The data presented here complements existing structural and theoretical studies on Cs+ complexation with calix[4] crown ethers.[8-10] By systematically exploring the coordination chemistry of elements from the first and second main groups of the periodic table, this work significantly enhances our understanding of the underlying interaction mechanisms. This knowledge can be used to improve existing extraction protocols or to develop new procedures for radionuclide decontamination and related applications.
- L. H. Delmau, P. V. Bonnesen, B. A. Moyer Hydrometallurgy 2004, 72, 9-19.
- B. A. Moyer, P. V. Bonnesen, L. H. Delmau, F. V. J. Sloop, N. J. Williams, J. F. J. Birdwell, D. L. Lee, R. A. Leonard, S. D. Fink, B. Peters, M. W. Geeting Development of the Next-Generation Caustic-Side Solvent Extraction (NG-CSSX) Process for Cesium Removal from High-Level Tank Waste Proceedings of the Waste Management Symposia, Phoenix, AZ, USA, 2011.
- R. A. Leonard, S. B. Aase, H. A. Arafat, C. Conner, D. B. Chamberlain, J. R. Falkenberg, M. C. Regalbuto, G. F. Vandegrift Solvent Extr. Ion Exch. 2003, 21, 505-526.
- D. D. Walker, M. A. Norato, S. G. Campbell, M. L. Crowder, S. D. Fink, F. F. Fondeur, M. W. Geeting, G. F. Kessinger, R. A. Pierce Sep. Sci. Technol. 2005, 40, 297-309.
- B. D. Roach, N. J. Williams, B. A. Moyer Solvent Extr. Ion Exch. 2015, 33, 576-591.
- M. Simonnet, T. Sittel, P. Weßling, A. Geist Energies 2022, 15, 7724.
- T. Sittel, K. Becker, A. Geist, P. J. Panak Solv. Extr. Ion Exch. 2024, 42, 118-132.
- P. Thuéry, M. Nierlich, C. Bressot, V. Lamare, J.F. Dozol, Z. Asfari, J. Vicens, J. Inclusion Phenom. Mol. Recognit. Chem. 1995, 23, 305-312.
- J. Kříž, J. Dybal, E. Makrlík, P. Vaňura, B. A. Moyer J. Phys. Chem. B 2011, 115, 7578-7587.
- V. Kumar, J. N. Sharma, P. V. Achuthan, D. K. Singh, S. M. Ali RSC Adv. 2016, 6, 47120-47129.
- Abstract category selection: