Oral Presentation International Solvent Extraction Conference 2025
Understanding the mechanisms of extraction of undesirable elements (Sr and Zr) by diglycolamides (122499)
Separation of Am(III) from the PUREX (Plutonium-Uranium Recovery by Extraction) raffinate can be achieved thanks to new processes such as the AmSel process, which is under development in Europe (Figure 1).
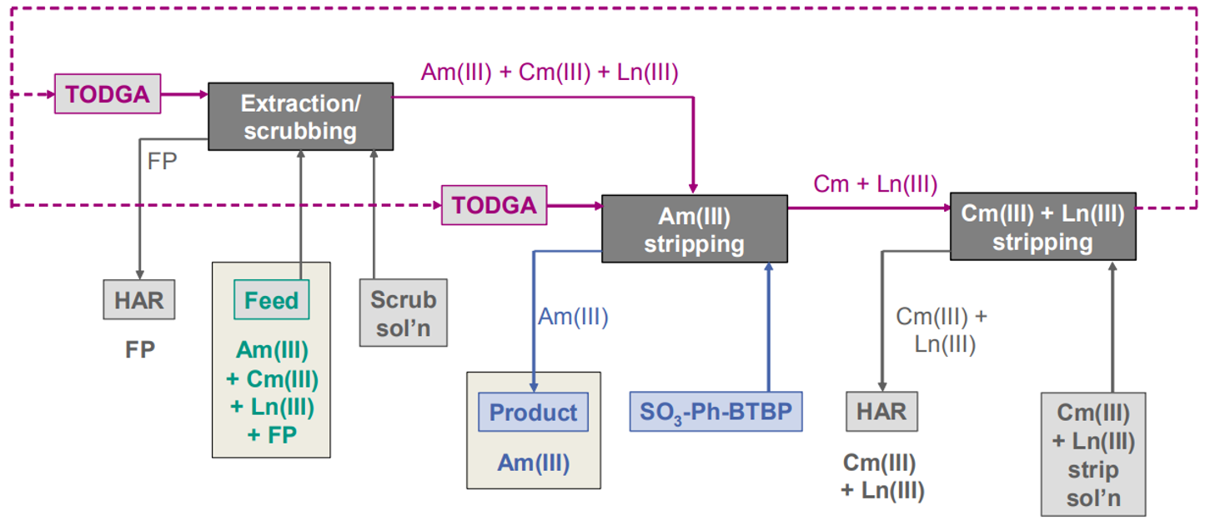
Figure 1: AmSel process diagram
The strategy relies on the co-extraction of An(III) and Ln(III) from the PUREX raffinate using a peculiar diglycolamide (DGA), N,N,N’,N’-tetraoctyl-diglycolamide (TODGA), followed by the subsequent selective stripping of Am(III) by a hydrosoluble ligand. DGAs have already been intensively studied for the separation of valence III elements, namely Am, Cm, and Ln, from fission products (FPs). However, DGAs, and in particular TODGA, lack selectivity, and some interfering elements are co-extracted, notably Sr(II) and Zr(IV) [1-2].
As of now, the extraction mechanism for these unwanted elements remains unknown.
Some articles have shown that the extraction of Sr(II) can be prevented depending on the alkyl chain length of the substituents [3]. They claim that shorter chains induce fewer aggregates, leading to a reduced extraction of Sr(II). Our own experiments with dimethyl-dioctyldiglycolamide (DMDODGA), which has 2 methyl chains instead of octyl, are in good agreement with a decreased Sr distribution ratio as compared to TODGA.
Furthermore, another set of experiments have shown that increasing the 1-octanol content in the organic phase decreases the extraction of Sr(II) (Figure 2).
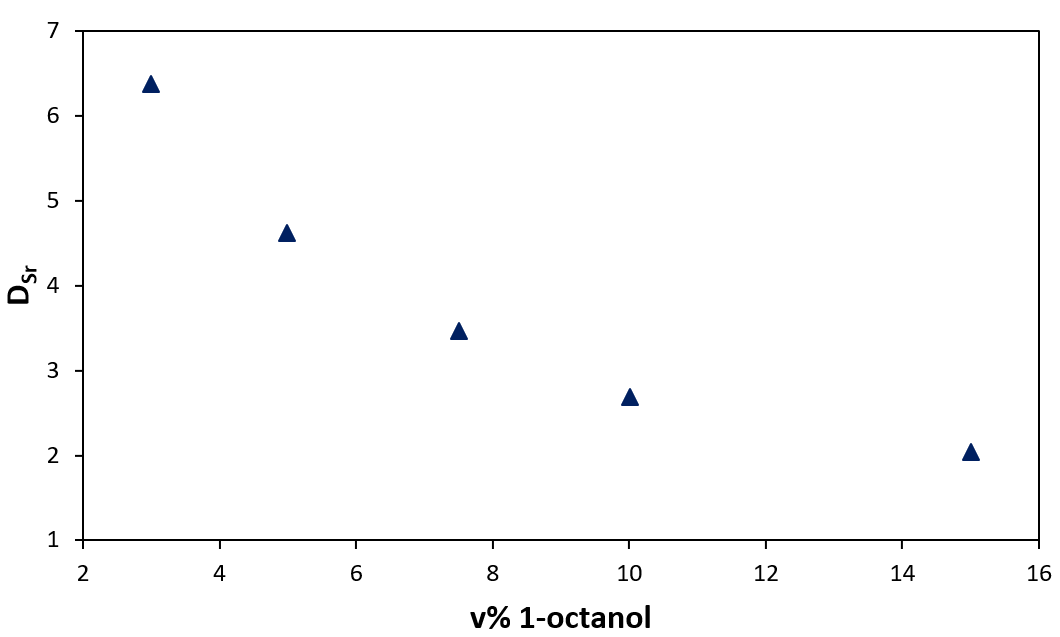
Figure 2: Sr(II) distribution coefficient as a function of 1-octanol volumic percentage
The presence of 1-octanol is known to prevent the formation of a third phase by limiting the growth of supramolecular aggregates. The Sr(II) extraction is thus reduced when the aggregation growth is limited. Both pieces of information suggest that the extraction of Sr(II) depends on the supramolecular assembly of the extractants.
Regarding Zr(IV), it has been identified as a major interfering element during the separation of An(III) for the treatment of high-level liquid waste (HLLW). Prathibha et al. [4] studied Zr(IV) extraction by DGAs from a nitric acid medium. A dynamic light scattering (DLS) study was carried out to analyze DGA aggregation in the presence of Zr(IV). The solvent system composed of 0.1 M TODGA and 0.25 M HDEHP (bis(-2-ethylhexyl)phosphoric acid) in n-dodecane showed a low tendency to aggregation and a high Zr(IV) loading capacity. The effect of a water-soluble complexing agent, namely trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid (CyDTA), was also investigated in order to suppress the co-extraction of Zr(IV) in the organic phase.
Based on these differents studies, we aim to correlate the contribution of the DGA aggregation to the extraction efficiency of Sr(II) and Zr(IV). Several key parameters influencing the extraction of Sr(II) and Zr(IV) mechanisms have been considered in this study to optimize the design of the Am separation process using DGAs: the variation of DGAs, nitric acid, and FPs concentrations, and the variation of the modifier content. This will draw a global picture of the outer-sphere interactions likely to influence the extraction of Sr(II) and Zr(IV) by DGAs.
Current state-of-the-art provides no direct correlation between the aggregation behavior and FPs co-extraction. The complementary techniques of SANS (Small-Angle Neutron Scattering) and SAXS (Small-Angle X-ray Scattering) could potentially assist in identifying one [5]. These analyses are therefore important to propose efficient and selective formulations, in order to optimize the molecular structure of new extracting systems.
Overall, the different results should provide a better understanding of the mechanisms of solvent extraction of Sr and Zr by DGA molecules, which could be incorporated into future process flowsheets. This will ultimately allow to establish the best formulation for the separation of f elements from PUREX raffinate in order to simplify the process.
- [1] Wagner, C.; Müllich, U.; Geist, A.; Panak, P. J., Selective Extraction of Am(III) from PUREX Raffinate: The AmSel System, Solvent Extraction and Ion Exchange, 2016, 34, 103.
- [2] Sasaki, Y., Tsubata, Y., Kitatsuji, Y., Sugo, Y., Shirasu, N., Morita, Y., Kimura, T., Extraction Behavior of Metal Ions by TODGA, DOODA, MIDOA, and NTAamide Extractants from HNO3 to n-dodecane, Solvent Extraction and Ion Exchange, 2013, 31 (4), 401–415.
- [3] Xu, Y., Gao, Y., Zhou, Y., Fan, C., Hou, H., Zhang, M., Extraction Behavior of Strontium from Nitric Acid Medium with N,N’-Dimethyl-N,N’-Dioctyldiglcolamide, Solvent Extraction and Ion Exchange, 2017, 35 (7), 507–518.
- [4] Prathibha, T., Rama Swami K., Suneesh A.S., Robert Selvan B., Sriram S., Venkatesan K.A., Extraction and Aggregation Behaviour of Zr(IV) in Diglycolamide Solvents during the Treatment of High-Level Liquid Waste Solution Arising from Metallic Fuel Reprocessing, Journal of Molecular Liquids, 2021, 340,117236.
- [5] Bourgeois D., El Maangar A., Dourdain S., Importance of weak interactions in the formulation of organic phases for efficient liquid/liquid extraction of metals, Current Opinion in Colloid & Interface Science, 2020, 46:36–51.
- Abstract category selection: