Oral Presentation International Solvent Extraction Conference 2025
Selective extraction of platinum group metals from a spent automotive catalyst leachate using benzoylpyridine-based hydrophobic deep eutectic solvents (121485)
The development of alternative solvents that exhibit environmental compatibility and excellent extraction performance is key to making solvent extraction more sustainable. Ionic liquids (ILs) have garnered considerable attention in this context due to their high extraction capabilities; nevertheless, their physical properties—particularly high viscosity—pose significant challenges to practical implementation. Deep eutectic solvents (DESs) have attracted attention as alternative solvents for metal extraction. A DES is a mixture of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) that forms a liquid at room temperature. Due to the hydrogen bonding interactions between the HBD and HBA, characteristic properties such as low volatility and high solubility are exhibited. However, due to the limited molecular diversity of hydrophobic DESs, there have been few studies on the separation of platinum group metals (PGMs) from automotive exhaust catalysts.
In this study, we prepared novel hydrophobic DESs containing benzoylpyridine as an effective extractant for PGMs. 2-, 3-, and 4-benzoylpyridine (BP) as HBAs were used to prepare DESs with thymol (Thy) as the HBD. The impact of BP isomerism on the physical properties and extraction performance was studied. The BP-based DESs were utilized for the extraction of platinum, palladium, and rhodium from a simulated leachate of spent automotive exhaust catalysts.
The solid-liquid equilibrium phase diagrams of the 2-, 3-, and 4-BP/Thy systems were estimated using the COSMO-RS method. These DESs exhibited a wide liquid window and could be employed as extraction solvents at a molar ratio of HBD:HBA = 1:1. The viscosities of the DESs were 42.1, 158.6, and 143.8 mPa·s, respectively, which are relatively low compared to previously reported hydrophobic ILs and DESs. These BP-based DESs show potential for practical use in PGM recycling.
The simulated automotive catalyst leachate contained Pt, Pd, and Rh along with impurity metals such as Fe, Zn, Mg, and Al. A typical PGM extractant, methyltrioctylammonium chloride (TOMAC), was used to prepare a DES for PGM extraction. The TOMAC/Thy DES demonstrated high extraction efficiency for Pt and Pd; however, poor extraction of Rh and the co-extraction of Fe and Zn hindered its applicability in the PGM recycling process. According to our optimization study, the 4-BP/Thy DES exhibited superior extraction performance for PGMs while effectively separating them from Fe, Mg, and other impurity metals at an aqueous HCl concentration of 3 mol/L. The remarkable suppression of Fe extraction is an intriguing feature that is not observed in conventional DESs. The co-extracted Zn could be selectively removed from the DES by scrubbing with 0.1 mol/L HCl without significant loss of PGMs. Rh and Pt could be selectively stripped using aqueous ammonium nitrate and thiourea solutions, respectively. The complete PGM separation process, including the reuse of the DES, will be presented at the conference.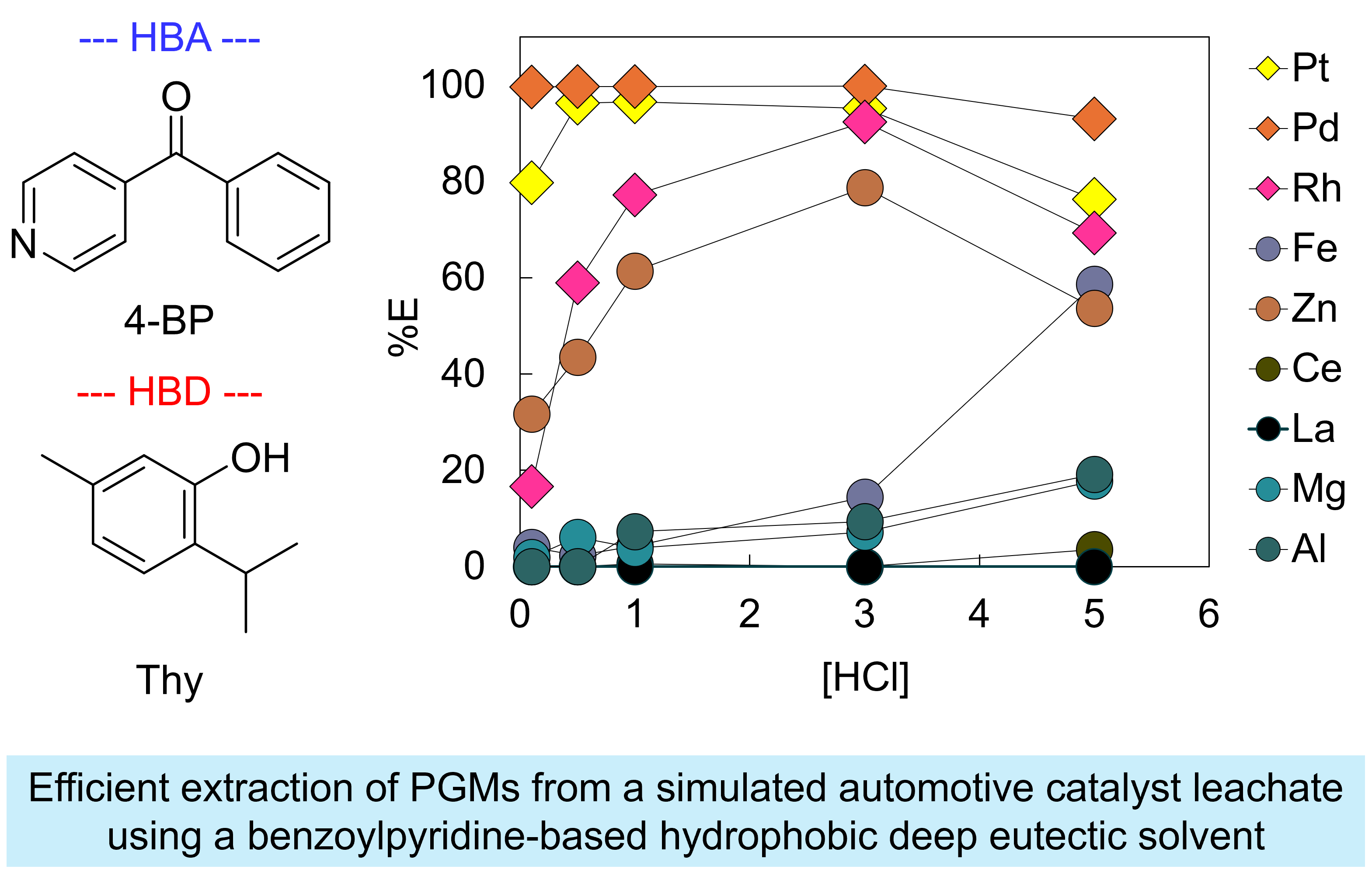
- Abstract category selection: