Plenary Presentation International Solvent Extraction Conference 2025
Hydrophobic Deep Eutectic Solvents for high-efficient nickel recovery from Nickel Laterite Ore (122641)
Introduction:
Ni ore resources primarily consist of laterite, magmatic sulfide, and hydrothermal deposits, with estimated reserves of 190, 124, and 35 million metric tons, respectively. Although laterite is abundant in the Earth’s crust, magmatic sulfide is currently the dominant ore used in the Ni industry. However, laterite has gained attention because of increasing concerns over the depletion of magmatic sulfide resources. Laterite is classified into two phases, limonite and saprolite. Limonite is typically processed using hydrometallurgical methods, such as high-pressure acid leaching and atmospheric leaching, producing high-purity Ni products like nickel sulfate and nickel matte for LIB applications. In contrast, saprolite is generally processed using pyrometallurgical methods, such as the rotary kiln-electric furnace process, yielding Ni alloys like ferronickel for the steel industry. Thus, saprolite has not been used as a resource in the LiB industry. Considering the increasing demand for LiBs, producing high-purity Ni products from saprolite presents a promising opportunity to establish a new Ni supply route, contributing to the growing Ni demand.
In recent years, deep eutectic solvents (DESs) have attracted attention as emerging solvents. DESs are obtained by mixing a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) in a specific ratio. Various chemical properties can be imparted to DESs depending on the HBA and HBD combination, making them highly designable and applicable in various fields such as hydrometallurgy and nanomaterials. Metal recovery methods using water-immiscible non-aqueous solvents, such as DESs, are referred to as solvometallurgy or ionometallurgy, and extensive research has been conducted on the recovery of lithium (Li), Ni, cobalt (Co), and manganese (Mn) from spent LiBs. However, reports on the use of DESs for metal recovery from primary resources like metal ores remain limited. By incorporating molecules with a high affinity for specific metals into HDESs, selective extraction of the target metals can be achieved. Consequently, HDES leaching offers significant advantages over acid leaching, including the reduction of acidic wastewater contaminated with hazardous metals and the ability to perform direct stripping from the HDES without requiring a separate extraction step.
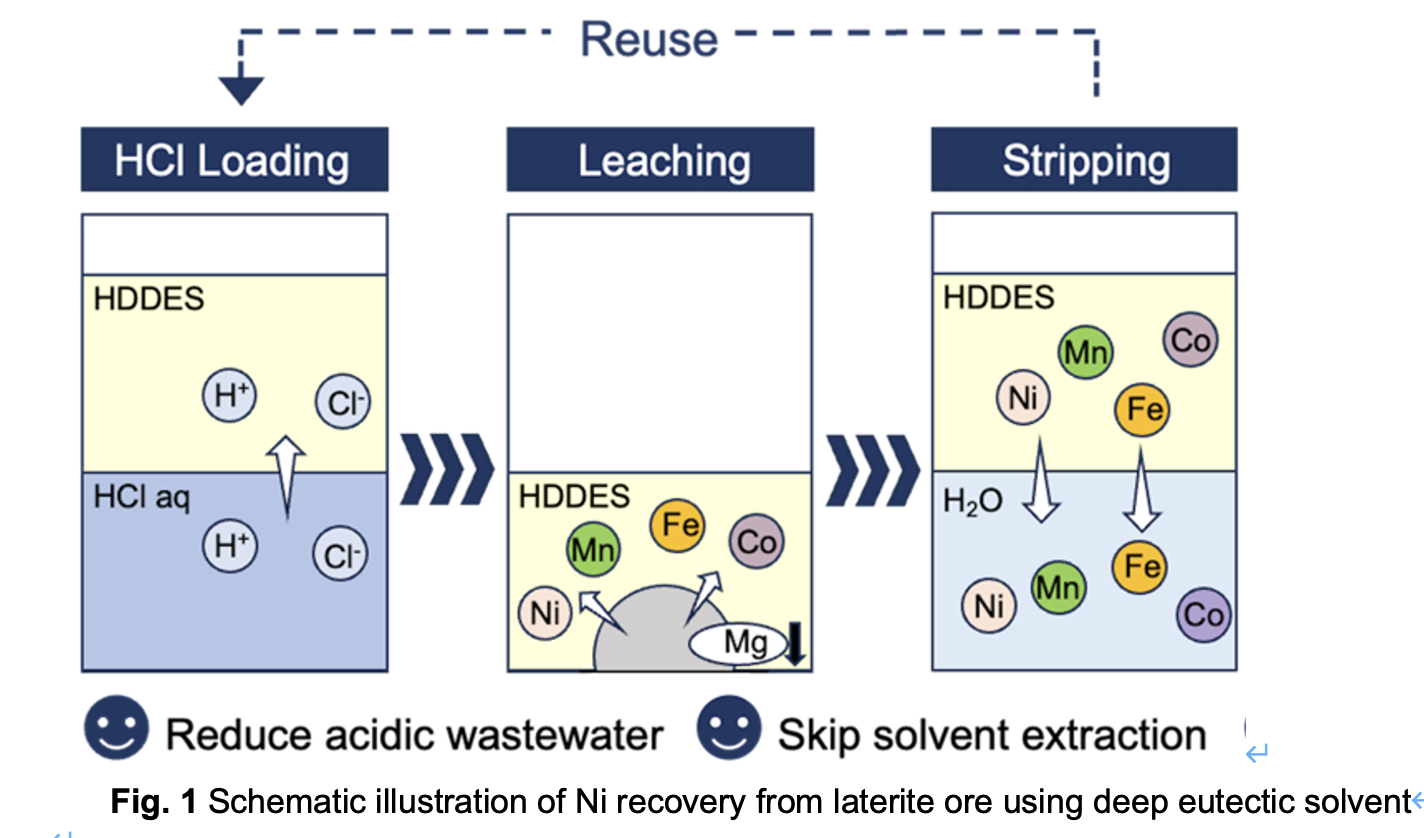
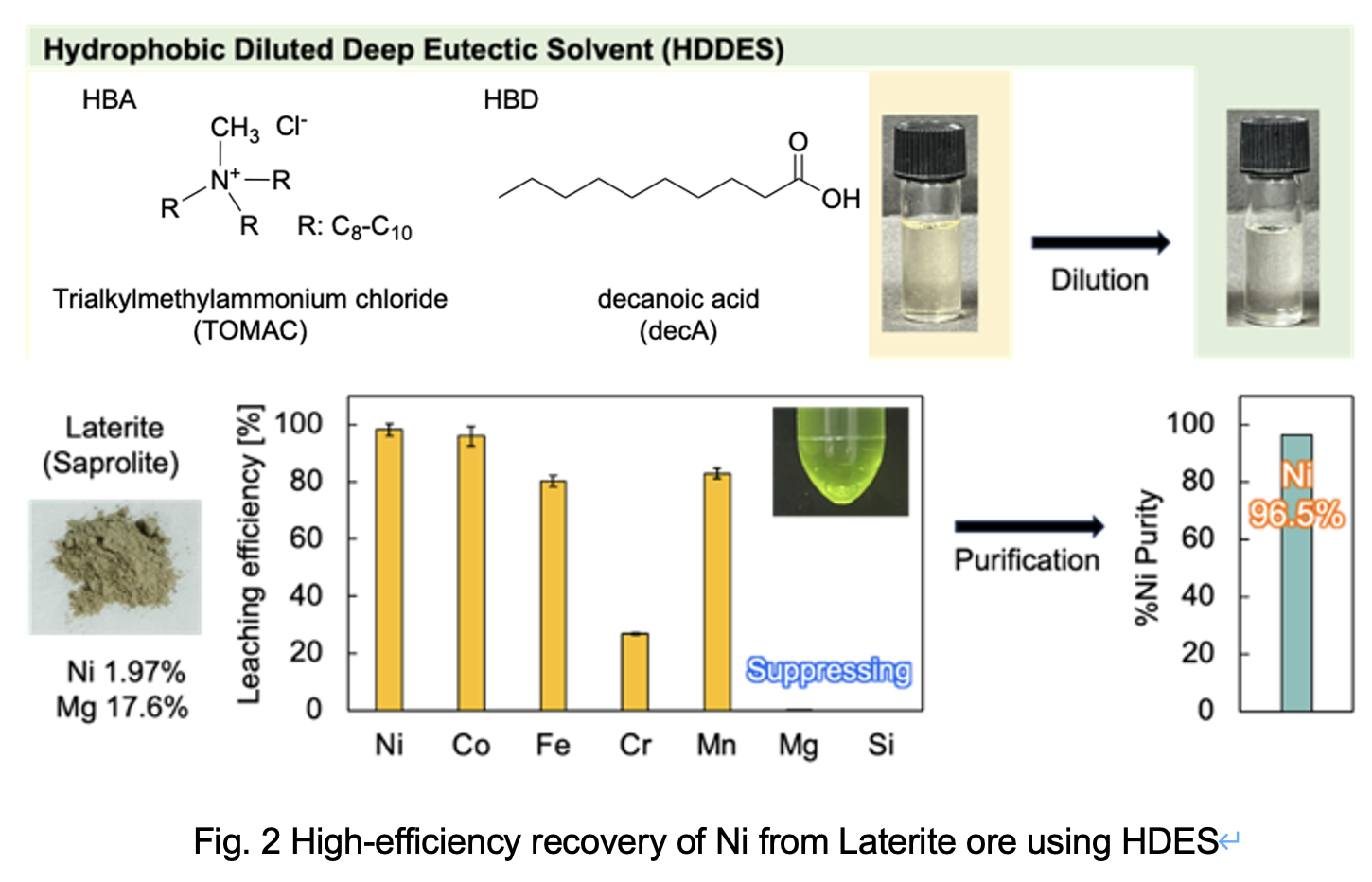
Notably, hydrophilic DESs can be diluted with water, maintaining their characteristics while reducing viscosity and improving cost-effectiveness, considering that diluents are typically inexpensive. Therefore, to overcome the challenges in industrial applications, the present study proposes a high-purity Ni recovery process with improved operability by incorporating industrial diluents into the HDES, a method that has not been previously reported. To perform the Ni recovery process illustrated in Fig. 1, HDES was prepared using trialkylmethylammonium chloride (TOMAC) as the HBA and decanoic acid (decA) as the HBD. All leached metals were then recovered in the aqueous phase by stripping.
Results:
In this study, an HDES was diluted with an organic solvent to address the industrial limitation of high-viscosity DESs and employed for the first time in nickel recovery from saprolite ore. Specifically, the HDDES’s low viscosity enhances operability and shortens leaching time, advancing their industrial applicability. The HDDES was prepared by diluting an HDES composed of TOMAC as the HBA and decA as the HBD with the industrial diluent Swasol 1800. When loaded with hydrochloric acid, HDDES demonstrated comparable Ni leaching performance to conventional sulfuric acid leaching systems while significantly suppressing Mg leaching. Subsequently, Ni was stripped into the aqueous phase along with other metals and further purified. As a result, impurities were selectively removed via precipitation, leading to the recovery of a Ni solution with a purity of 97% (Fig. 2). Furthermore, the HDDES exhibited excellent reusability over five leaching cycles. These results not only demonstrate the sustainability of the HDDES-based metal recovery process but also highlight the potential of saprolite as a Ni source for LiB applications.
- Abstract category selection: